
Oregon Medicaid Pharmaceutical Services
Prior Authorization Criteria
HEALTH SYSTEMS DIVISION
Prior authorization (PA)
criteria for fee-for-service
prescriptions for Oregon Health Plan
clients
July 1, 2017

Oregon Medicaid PA Criteria 2 July 1, 2017
Contents
Contents ................................................................................................................................................................ 2
Introduction........................................................................................................................................................... 6
About this guide ......................................................................................................................................... 6
How to use this guide ................................................................................................................................. 6
Administrative rules and supplemental information .................................................................................. 6
Update information .............................................................................................................................................. 7
Effective July 1, 2017 ................................................................................................................................ 7
Substantive updates and new criteria ............................................................................................. 7
Clerical changes ............................................................................................................................. 7
General PA information ....................................................................................................................................... 8
Overview .................................................................................................................................................... 8
Drugs requiring PA - See OAR 410-121-0040 for more information ....................................................... 8
DUR Plus review ....................................................................................................................................... 8
How to request PA ..................................................................................................................................... 9
For prescriptions and oral nutritional supplements ........................................................................ 9
For emergent or urgent prescriptions that require PA ................................................................... 9
For diabetic supplies (lancets, test strips, syringe and glucose monitor supplies) ......................... 9
Client hearings and exception requests .................................................................................................... 10
DMAP 3978 - Pharmacy Prior Authorization Request............................................................................ 10
Information needed to request PA ............................................................................................... 10
PA criteria for fee-for-service prescriptions .................................................................................................... 13
About the PA criteria ............................................................................................................................... 13
Contact for questions about PA policy .................................................................................................... 13
Attention Deficit Hyperactivity Disorder (ADHD) Safety Edit ......................................................................... 14
Analgesics, Non-Steroidal Anti-Inflammatory Drugs ...................................................................................... 17
Antiemetics ......................................................................................................................................................... 18
Antifungals .......................................................................................................................................................... 20
Oregon Medicaid PA Criteria 3 July 1, 2017
Antihistamines .................................................................................................................................................... 24
Antimigraine - Triptans ...................................................................................................................................... 26
Anti-Parkinson’s Agents .................................................................................................................................... 29
Antiplatelets ........................................................................................................................................................ 30
Antivirals for Herpes Simplex Virus ................................................................................................................. 32
Antivirals - Influenza .......................................................................................................................................... 34
Becaplermin (Regranex
®
) .................................................................................................................................. 36
Benign Prostatic Hypertrophy (BPH) Medications .......................................................................................... 37
Benzodiazepines ................................................................................................................................................ 39
Biologics for Autoimmune Diseases ................................................................................................................ 40
Bone Resorption Inhibitors and Related Agents............................................................................................. 45
Botulinum Toxins ............................................................................................................................................... 47
Buprenorphine and Buprenorphine/Naloxone ................................................................................................ 52
Calcium and Vitamin D Supplements ............................................................................................................... 55
Clobazam............................................................................................................................................................. 56
Codeine ............................................................................................................................................................... 57
Conjugated Estrogens/Bazedoxifene (Duavee
®
) ............................................................................................. 58
Cough and Cold Preparations ........................................................................................................................... 60
Cysteamine Delayed-release (PROCYSBI
®
) ..................................................................................................... 61
Daclizumab (Zinbryta™) .................................................................................................................................... 62
Dalfampridine...................................................................................................................................................... 63
Dispense as Written-1 (DAW-1) Reimbursement Rate .................................................................................... 65
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors........................................................................................................ 67
Dronabinol (Marinol®) ........................................................................................................................................ 68
Droxidopa (Northera®) ....................................................................................................................................... 70
Drugs for Constipation ...................................................................................................................................... 72
Drugs Selected for Manual Review by Oregon Health Plan ........................................................................... 74
Drugs for Non-funded Conditions .................................................................................................................... 75
Erythropoiesis Stimulating Agents (ESAs) ...................................................................................................... 76
Estrogen Derivatives .......................................................................................................................................... 78
Exclusion List ..................................................................................................................................................... 80
Fidaxomicin (Dificid®) ....................................................................................................................................... 85
Glucagon-like Peptide-1 (GLP-1) Receptor Agonists...................................................................................... 86
Gonadotropin-Releasing Hormone (GnRH) Analogs ...................................................................................... 88
Agents for Gout .................................................................................................................................................. 89
Growth Hormones .............................................................................................................................................. 91
Oregon Medicaid PA Criteria 4 July 1, 2017
Hepatitis B Antivirals ......................................................................................................................................... 94
Hepatitis C Direct-Acting Antivirals .................................................................................................................. 96
Hydroxyprogesterone caproate ...................................................................................................................... 102
Idiopathic Pulmonary Fibrosis (IPF) Agents .................................................................................................. 104
Inhaled Corticosteroids (ICS) .......................................................................................................................... 105
Initial Pediatric SSRI Antidepressant – Daily Dose Limit ............................................................................. 107
Insulins .............................................................................................................................................................. 109
Intranasal Allergy Drugs .................................................................................................................................. 110
Ivabradine (Corlanor
®
) ..................................................................................................................................... 112
Long-acting Beta-agonists (LABA) ................................................................................................................. 114
Long-acting Beta-agonist/Corticosteroid Combination (LABA/ICS) ........................................................... 116
Long-acting Muscarinic Antagonist/Long-acting Beta-agonist Combination (LAMA/LABA) ................... 118
Lidocaine Patch ................................................................................................................................................ 120
Low Dose Quetiapine ....................................................................................................................................... 122
Milnacipran........................................................................................................................................................ 124
Mipomersen and Lomitapide ........................................................................................................................... 125
Modafinil / Armodafinil ..................................................................................................................................... 126
Monoclonal Antibodies for Severe Asthma ................................................................................................... 130
Oral Multiple Sclerosis Drugs ......................................................................................................................... 133
Multivitamins..................................................................................................................................................... 136
New Drug Policy ............................................................................................................................................... 138
Nusinersen ........................................................................................................................................................ 139
Nutritional Supplements (Oral Administration Only) .................................................................................... 140
Obeticholic Acid (Ocaliva
®
) ............................................................................................................................. 145
Ocular Vascular Endothelial Growth Factors ................................................................................................ 147
Omega-3 Fatty Acids ........................................................................................................................................ 148
Opioid Analgesics ............................................................................................................................................ 150
Oral Cystic Fibrosis Modulators ..................................................................................................................... 156
Oxazolidinone Antibiotics ............................................................................................................................... 162
Palivizumab (Synagis
®
) .................................................................................................................................... 163
Patiromer ........................................................................................................................................................... 168
PCSK9 Inhibitors .............................................................................................................................................. 170
Preferred Drug List (PDL) – Non-Preferred Drugs in Select PDL Classes .................................................. 173
Peginterferon Beta-1a (Plegridy®) .................................................................................................................. 174
Pegylated Interferons and Ribavirins ............................................................................................................. 175
Phosphate Binders ........................................................................................................................................... 180
Oregon Medicaid PA Criteria 5 July 1, 2017
Pimavanserin (Nuplazid™) Safety Edit........................................................................................................... 181
Pregabalin ......................................................................................................................................................... 182
Proton Pump Inhibitors (PPIs) ........................................................................................................................ 184
Oral/Inhaled Pulmonary Arterial Hypertension Agents ................................................................................ 187
Injectable Pulmonary Arterial Hypertension Agents (IV/SC) ........................................................................ 189
Repository Corticotropin Injection ................................................................................................................. 190
Repository Corticotropin Injection (Acthar Gel®) ......................................................................................... 192
Rifaximin (Xifaxan®) ........................................................................................................................................ 194
Risperdal
®
Consta
®
Quantity Limit ................................................................................................................. 195
Roflumilast ........................................................................................................................................................ 196
Sacubitril/Valsartan (Entresto™) .................................................................................................................... 197
Sapropterin ....................................................................................................................................................... 199
Sedatives ........................................................................................................................................................... 201
Sodium-Glucose Cotransporter-2 Inhibitors (SGLT-2 Inhibitors) ................................................................ 203
Skeletal Muscle Relaxants ............................................................................................................................... 205
Smoking Cessation .......................................................................................................................................... 207
Tesamorelin (Egrifta
®
) ...................................................................................................................................... 209
Testosterone ..................................................................................................................................................... 210
Topical Antipsoriasis Drugs ............................................................................................................................ 212
Topiramate ........................................................................................................................................................ 214
Oregon Medicaid PA Criteria 6 July 1, 2017
Introduction
About this guide
The Oregon Medicaid Pharmaceutical Services PA Criteria is designed to assist the following
providers:
Prescribing providers seeking approval of fee-for-service (FFS, or “open card”)
prescriptions for Oregon Health Plan (OHP) clients
Pharmacies filling FFS prescriptions for OHP clients
How to use this guide
The table of contents is not interactive. When viewing this guide electronically, do the
following to quickly access PA criteria:
Click the Bookmarks button in your PDF viewer to view the bookmarks in this guide.
Click on the bookmark you wish to view to go to that page.
A plus sign next to the bookmark name means there are additional items within that
bookmark. Click the plus sign to see the additional bookmarks.
To turn pages within the PDF, use the arrow buttons (normally located at the top or
bottom of your PDF viewer).
Administrative rules and supplemental information
Use this guide with the Pharmaceutical Services provider guidelines (administrative rules and
supplemental information), which contain information on policy and covered services specific
to your provider type.
You can find these guidelines at
www.oregon.gov/OHA/healthplan/Pages/Pharmacy-policy.aspx

Oregon Medicaid PA Criteria 7 July 1, 2017
Update information
Effective July 1, 2017
The Health Systems Division made substantive changes to listed criteria, deleted criteria, and
made minor, non-substantive formatting updates to the entire guide.
Substantive updates and new criteria
Hepatitis C Direct-acting antivirals
Ocular Vascular Endothelial Growth Factors
Proton Pump Inhibitors
Clerical changes
Sacubitril/valsartan (Entresto)
Sedatives
Antimigraine - Triptans
For questions, contact the Division’s Pharmacy Program at dmap.rxquestions@state.or.us.

Oregon Medicaid PA Criteria 8 July 1, 2017
General PA information
Overview
For drugs that require PA on Point of Sale (POS) claims:
A new evaluation feature of the Oregon Medicaid POS system, DUR Plus, reviews
incoming POS claims and issues PA when the drug meets appropriate clinical criteria.
For drugs that do not pass DUR Plus review, pharmacies must contact the prescribing
provider, who then requests PA from the Oregon Pharmacy Call Center.
Drugs requiring PA - See OAR 410-121-0040 for more information
The Division may require PA for individual drugs and categories of drugs to ensure that the
drugs prescribed are indicated for conditions funded by OHP and consistent with the Prioritized
List of Health Services and its corresponding treatment guidelines (see OAR 410-141-0480 and
410-141-0520).
DUR Plus review
The Oregon Medicaid POS system initially evaluates incoming pharmacy claims for basic edits
and audits. If the drug on the claim requires PA and requires DUR Plus evaluation, the claim
passes through a series of clinical criteria rules to determine whether DUR Plus can issue PA
and allow dispensing the drug to the client.
DUR Plus checks the current drug claim as well as the client’s medical and claims history for
the appropriate criteria.
If suitable criteria are found, a prior authorization will be systematically created, applied
to the claim, and the claim will be paid. This interactive process occurs with no
processing delays and no administrative work for the pharmacy or prescribing provider.
If all criteria are not met, the claim will be denied and PA will be required. The prescriber
will be responsible for requesting PA, using procedures outlined in OAR 410-121-0060.

Oregon Medicaid PA Criteria 9 July 1, 2017
How to request PA
For prescriptions covered by the client’s coordinated care organization (CCO), contact the CCO
for their PA procedures.
For prescriptions covered by OHA on a fee-for-service (“open card”) basis, use the following
contact information:
For prescriptions and oral nutritional supplements
The Oregon Pharmacy Call Center is available 24 hours per day, seven days a week, 365 days a
year and processes PA requests within 24 hours. When calling in a PA request, have the
diagnosis code ready.
Phone: 888-202-2126
Fax: 888-346-0178
Refer to PA procedures outlined in OAR 410-121-0060.
For emergent or urgent prescriptions that require PA
The Oregon Pharmacy Call Center may authorize up to a 96 hour emergency supply for drugs
that require PA, but have no PA on file. Refer to 410-121-0060(4) Emergency Need.
The Pharmacist may request an emergent or urgent dispensing from the Pharmacy Call Center
when the client is eligible for covered fee-for-service drug prescriptions.
a) Clients who do not have a PA pending may receive an emergency dispensing for a 96-
hour supply.
b) Clients who do have a PA pending may receive an emergency dispensing for up to a
seven-day supply.
For diabetic supplies (lancets, test strips, syringe and glucose monitor supplies)
Diabetic supplies in excess of OHA’s utilization guidelines require PA from the Division:
Health Systems Division – Provider Clinical Support Unit
500 Summer St NE, E44
Salem, OR 97301-1078
503-945-6821 (direct)
800-642-8635 (in-state only)
Use the MSC 3971 form to submit PA requests. Fax the completed form using an EDMS
Coversheet (MSC 3970) to one the following fax numbers:
Routine requests: 503-378-5814
Immediate/urgent requests: 503-378-3435

Oregon Medicaid PA Criteria 10 July 1, 2017
Client hearings and exception requests
For any PA requests that are denied due to OHA criteria not being met, the right of a client to
request a contested case hearing is otherwise provided by statute or rule, including OAR 410-
141-0264(10).
This rule describes when a client may request a state hearing. Clients may request a
hearing based upon information included in the PA denial notice.
Information on how to file an appeal is attached to all PA notices to clients and providers
from the Oregon Pharmacy Call Center.
Providers may contact Provider Services at 800-336-6016 to file an exception request on a PA
denial. For information regarding OAR 410-120-1860, refer to the Division’s General Rules at
www.oregon.gov/OHA/healthplan/pages/general-rules.aspx
DMAP 3978 - Pharmacy Prior Authorization Request
This form is the paper option for submitting pharmacy PA requests. Prescribers should submit
their PA requests for fee-for-service prescriptions and oral nutritional supplements with
required documentation to the Oregon Pharmacy Call Center at 888-346-0178.
This form does not require an EDMS Coversheet. This form is also available on the DHS/OHA
website at https://apps.state.or.us/Forms/Served/OE3978.pdf.
Information needed to request PA
Complete the form as follows. The Oregon Pharmacy Call Center may ask for some or all of the
following information, depending upon the class of the drug requested:
DMAP 3978
section
Information needed
Section I:
Requesting provider name and National Provider Identifier
FQHC/RHC and AI/AN providers - Also enter the pharmacy or clinic NPI for
your facility
Section II
Type of PA Request: Mark “Pharmacy”
FQHC/RHC and AI/AN providers -Mark “Other,” followed by provider type
(FQHC, RHC, IHS or Tribal 638)
Section III:
Client name and recipient ID number
Section IV:
Diagnosis code
Section V:
Drug name, strength, size and quantity of medication
Participating pharmacy: Include the dispensing pharmacy’s name and phone
number (if available)
Section VI:
Date of PA Request Begin and End Dates of Service
Section VII:
Complete for EPIV and oral nutritional supplements only
Section VIII:
Complete for oral nutritional supplements only

Oregon Medicaid PA Criteria 11 July 1, 2017

Oregon Medicaid PA Criteria 12 July 1, 2017

Oregon Medicaid PA Criteria 13 July 1, 2017
PA criteria for fee-for-service prescriptions
About the PA criteria
The following pages include specific drugs, goals or directives in usage, length of authorization,
covered alternatives, approval criteria and more.
The Division’s prior authorization policy is reviewed by the Oregon Pharmacy and Therapeutic
Committee (P&T Committee) and is subject to the Oregon Administrative Rule writing process.
To learn more about the P&T Committee, please visit the Web page at
http://www.oregon.gov/OHA/pharmacy/Pages/pt-commitee.aspx.
For summaries of P&T Committee recommendations approved by OHA for policy
implementation, view the OHA Recommendations posted at
http://www.oregon.gov/oha/pharmacy/Pages/pt-committee.aspx.
Contact for questions about PA policy
For general questions about the Division’s prior authorization policy for fee-for-service
prescriptions, please contact:
Roger A. Citron, RPh
OSU College of Pharmacy
Drug Use Research & Management at
OHA Health Systems Division
500 Summer Street NE, E-35
Salem, OR 97301-1079
roger.a.citron@state.or.us
Voicemail: 503-947-5220
Fax: 503-947-1119

Oregon Medicaid PA Criteria 14 July 1, 2017
Attention Deficit Hyperactivity Disorder (ADHD) Safety Edit
Goals:
Cover ADHD medications only for diagnoses funded by the OHP and medications consistent with
current best practices.
Promote care by a psychiatrist for patients requiring therapy outside of best-practice guidelines.
Promote preferred drugs in class.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs on the enforceable preferred drug list.
Regimens prescribed outside of standard doses and age range (Tables 1 and 2)
Non-standard polypharmacy (Table 3)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Table 1. FDA-approved and OHP-funded Indications.
STIMULANTS
NON-STIMULANTS
Indication
Methylphenidate
and derivatives
Amphetamine
and
derivatives
Atomoxetine
Clonidine ER
Guanfacine ER
ADHD
Age ≥6 years
Age ≥3 years
Age ≥6 years
Children age
6-17 years only
Children age
6-17 years only
Narcolepsy
Age ≥6 years
Age ≥6 years
Not approved
Not approved
Not approved
Table 2. Standard Age and Maximum Daily Doses.
Drug Type
Generic Name
Minimum
Age
Maximum
Age
Maximum Daily Dose (adults
or children <18 years of age
unless otherwise noted)
CNS Stimulant
amphetamine/dextroamphetamine
salts IR
3
40 mg
CNS Stimulant
amphetamine/dextroamphetamine
salts ER
6
60 mg
CNS Stimulant
dexmethylphenidate IR
6
20 mg
CNS Stimulant
dexmethylphenidate LA
6
40 mg for adults or
30 mg if age <18 years
CNS Stimulant
dextroamphetamine IR
6
40 mg
CNS Stimulant
dextroamphetamine LA
6
60 mg
CNS Stimulant
lisdexamfetamine
6
70 mg
CNS Stimulant
methamphetamine
6
17
not established
CNS Stimulant
methylphenidate IR
4
60 mg
CNS Stimulant
methylphenidate LA
6
72 mg
CNS Stimulant
methylphenidate transdermal
6
17
30 mg
Non-Stimulant
atomoxetine
6
100 mg
Non-Stimulant
clonidine LA
6
17
0.4 mg
Non-Stimulant
guanfacine LA
6
17
4 mg
Abbreviations: IR = immediate-release formulation; LA = long-acting formulation (extended-release, sustained-release, etc.)

Oregon Medicaid PA Criteria 15 July 1, 2017
Table 3. Standard Combination Therapy for ADHD
Age Group
Standard Combination Therapy
Age <6 years*
Combination therapy not recommended
Age 6-17 years*
1 CNS Stimulant Formulation (LA or IR) + Guanfacine LA
1 CNS Stimulant Formulation (LA or IR) + Clonidine LA
Age ≥18 years**
Combination therapy not recommended
Abbreviations: IR = immediate-release formulation; LA = long-acting formulation (extended-release, sustained-release, etc.)
* As recommended by the American Academy of Pediatrics 2011 Guidelines www.pediatrics.org/cgi/doi/10.1542/peds.2011-2654
**As identified by Drug Class Review: Pharmacologic Treatments for Attention Deficit Hyperactivity Disorder: Drug Effectiveness
Review Project, 2011.
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the treated diagnosis an OHP-
funded condition?
Yes: Go to #3
No: Pass to RPh. Deny; not
funded by OHP.
3. Is the requested drug on the PDL?
Yes: Go to #5
No: Go to #4
4. Will the prescriber consider a
change to a preferred agent?
Message:
Preferred drugs are evidence-based
reviewed for comparative
effectiveness and safety by the
Oregon Pharmacy & Therapeutics
(P&T) Committee.
Yes: Inform prescriber of
preferred alternatives
No: Go to #5
5. Is the request for an approved FDA
indication defined in Table 1?
Yes: Go to #6
No: Go to #9
6. Are the patient’s age and the
prescribed dose within the limits
defined in Table 2?
Yes: Go to #7
No: Go to #9
7. Is the prescribed drug the only
stimulant or non-stimulant filled in
the last 30 days?
Yes: Approve for up to
12 months
No: Go to #8
8. Is the multi-drug regimen
considered a standard combination
as defined in Table 3?
Yes: Approve for up to
12 months
No: Go to #9
Oregon Medicaid PA Criteria 16 July 1, 2017
Approval Criteria
9. Was the drug regimen developed
by, or in consultation with, a
psychiatrist, developmental
pediatrician, psychiatric nurse
practitioner, sleep specialist or
neurologist?
Yes: Document name
and contact information
of consulting provider
and approve for up to 12
months
No: Pass to RPh. Deny;
medical appropriateness.
Doses exceeding defined limits
or non-recommended multi-
drug regimens of stimulants
and/or non-stimulants are only
approved when prescribed by a
psychiatrist or in consultation
with a mental health specialist.
May approve continuation of
existing therapy once up to 90
days to allow time to consult
with a mental health specialist.
P&T Review: 5/16 (KK); 3/16 (AG); 5/14; 9/09; 12/08; 2/06; 11/05; 9/05; 5/05; 2/01; 9/00; 5/00
Implementation: 10/13/16; 7/1/16; 10/9/14; 1/1/15; 9/27/14; 1/1/10; 7/1/06; 2/23/06; 11/15/05

Oregon Medicaid PA Criteria 17 July 1, 2017
Analgesics, Non-Steroidal Anti-Inflammatory Drugs
Goal(s):
To ensure that non-preferred NSAIDs are used for conditions funded by the OHP.
Restrict ketorolac to short-term use (5-day supply every 60 days) per the FDA black boxed
warning.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred NSAIDs.
Ketorolac: Maximum of one claim per 60 days, with a maximum 20 tablets/5-day supply
(maximum 5-day supply every 60 days).
Preferred Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the diagnosis funded by the Oregon
Health Plan?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP
3. Is this a continuation of current therapy (i.e.
filled prescription within prior 90 days)?
Verify via pharmacy claims.
Yes: Document prior
therapy in PA record. Go
to #4.
No: Go to #5
4. Is request for more than a 5-day supply of
ketorolac within 60 days (200 mg total over
5 days for tablets, 630 mg total over 5 days
for the nasal spray)?
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Go to #5
5. Will the prescriber consider switching to a
preferred product?
Message:
Preferred products do not require PA or
copay.
Preferred products are evidence-based
reviewed for comparative effectiveness &
safety by the Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Approve for up to
12 months.
P&T Review: 3/16 (MH); 11/14; 9/13; 2/12; 9/09; 2/06
Implementation: 1/1/15, 1/1/14, 5/14/12, 1/1/10

Oregon Medicaid PA Criteria 18 July 1, 2017
Antiemetics
Goal(s):
Promote use of preferred antiemetics.
Restrict use of antiemetics for OHP-funded conditions.
Restrict inappropriate chronic use.
For patients receiving chemotherapy or radiation, approve a quantity sufficient for 3 days beyond
the duration of treatment.
Length of Authorization:
Up to 6 months, or variable depending on chemotherapy
Requires PA:
Non-preferred drugs
Preferred drugs when quantity limit exceeded (Table 1)
Table 1. Quantity Limits for Antiemetic Drugs.
Drug
Trade Name
Dose Limits
5-HT3 Receptor Antagonists
Ondansetron
Zofran, Zuplenz, generic formulations
12 doses/ 7 days
Dolasetron
Anzemet
1 dose/ 7 days
Granisetron
Sancuso transdermal
Generic oral
1 patch / 7 days
1 dose/ 7 days
Substance P/neurokinin 1 (NK1) Receptor Antagonists
Aprepitant
Emend
3 doses/ 7 days
Rolapitant
Varubi
1 dose/ 7 days
Substance P/neurokinin 1 (NK1) Receptor Antagonists and 5-HT3 Receptor Antagonists Combinations
Netupitant/palonosetron
Akynzeo
1 dose/ 7 days
Cannabinoid Receptor Agonist
Dronabinol
Marinol
2.5 mg and 5 mg = 3
doses/day
10 mg = 2 doses/day
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What is the diagnosis being
treated?
Record ICD10 Code.
2. Is the diagnosis funded by OHP?
Yes: Go to #3
No: Pass to RPh. Deny; not
funded by the OHP
3. Is the requested drug preferred?
Yes: Go to #5
No: Go to #4
Oregon Medicaid PA Criteria 19 July 1, 2017
4. Will the prescriber consider a
change to the preferred product?
Note:
Preferred products do not
require a PA unless they
exceed dose limits in Table 1.
Preferred products are
evidence-based reviewed for
comparative effectiveness and
safety by the Pharmacy and
Therapeutics Committee.
Yes: Inform prescriber of
covered alternatives in class
and dose limits. If dose
exceeds limits, go to #5.
No: Go to #5
5. Is the request for
doxylamine/pyridoxine (Diclegis
®
)
for pregnancy-related nausea or
vomiting?
Yes: Go to #6
No: Go to #7
6. Has the patient failed a trial of
pyridoxine?
Note:
Preferred pyridoxine products
do not require a PA and are
reviewed for comparative
effectiveness and safety by the
Pharmacy and Therapeutics
Committee.
Yes: Approve for up to 3
months
No: Pass to RPh; deny and
recommend a trial of
pyridoxine.
7. Is the request for dronabinol?
Yes: Go to #8
No: Go to #9
8. Does the patient have anorexia
associated with HIV/AIDS?
Yes: Approve for up to 6
months. Apply quantity limit
for drugs listed in Table 1.
No: Go to #9
9. Does the patient have a cancer
diagnosis AND receiving
chemotherapy or radiation?
Yes: Approve for 3 days
beyond length of
chemotherapy regimen or
radiation (not subject to
quantity limits)
No: Go to #10
10. Does patient have refractory
nausea/vomiting that has resulted
in hospitalizations or ED visits in
the past 6 months?
Yes: Approve for up to 6
months (not subject to
quantity limits)
No: Go to #11
11. Has the patient tried and failed, or
have contraindications, to at least 2
preferred antiemetics?
Yes: Approve for up to 6
months. Apply quantity limit
for drugs listed in Table 1.
No: Pass to RPh. Deny;
medical appropriateness.
Must trial at least 2
preferred antiemetics
P&T/DUR Review: 1/17 (DM); 1/16; 11/14; 9/09; 2/06; 2/04; 11/03; 9/03; 5/03; 2/03
Implementation: 4/1/17; 2/12/16; 1/1/15; 1/1/14; 1/1/10; 7/1/06; 3/20/06; 6/30/04; 3/1/04; 6/19/03; 4/1/03

Oregon Medicaid PA Criteria 20 July 1, 2017
Antifungals
Goal(s):
Approve use of antifungals only for OHP-funded diagnoses. Minor fungal infections of skin,
such as dermatophytosis and candidiasis are only funded when complicated by an
immunocompromised host.
Length of Authorization:
See criteria
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Table 1: Examples of FUNDED indications (1/1/15)
ICD-10
Description
B373
Candidiasis of vulva and vagina
B371
Candidiasis of the lung
B377
Disseminated Candidiasis
B375-376, B3781-3782, B3784-
3789
Candidiasis of other specified sites
B380-B384, B3889, B389
Coccidiomycosis various sites
B392-395, B399, G02, H32, I32,
I39, J17
Histoplamosis
B409,B410, B419, B480
Blastomycosis
B420-427, B429, B439, B449-450,
B457, B459, B469, B481-482,
B488, B49
Rhinosporidosis, Sporotrichosis, Chromoblastomycosis,
Aspergillosis, Mycotis Mycetomas, Cryptococcosis,
Allescheriosis, Zygomycosis, Dematiacious Fungal Infection,
Mycoses Nec and Nos
B488
Mycosis, Opportinistic
B4481
Bronchopulmonary Aspergillus, Allergic
N739-751, N759, N760-
N771(except N72)
Inflammatory disease of cervix vagina and vulva
L3019,L3029, L3039, L3049
Cellulitis and abscess of finger and toe
P375
Neonatal Candida infection
Table 2: Examples of NON-FUNDED indications (1/1/15)
ICD-10
Description
L2083, L210-211, L218-219, L303
Erythematosquamous dermatosis
L22
Diaper or napkin rash
L20.0-20.82, L20.84-20.89
Other atopic dermatitis and related conditions
L240-242, L251-255, L578, L579,
L230, L2381, L2481, L250, L252,
L258-259, L551-552 , L568, L589
Contact dermatitis and other eczema
L530-532, L510, L518-519, L52,
Erythematous conditions

Oregon Medicaid PA Criteria 21 July 1, 2017
L710-711, L718, L930, L932,
L490-L499, L26, L304, L538,
L920, L951, L982, L539
L438,L441-443, L449,L661
Lichen Planus
L700-702, L708
Rosacea or acne
B351
Tinea unguium (onychomycosis)
B360
Pityriasis versicolor
B362
Tinea blanca
B363
Black piedra
B368, B369
Mycoses, superficial
B372
Cutaneous candidiasis
B379
Candidiasis, unspecified
R21
Rash and other nonspecific skin eruption
Table 3: Criteria driven diagnoses (1/1/15)
ICD-10
Description
B350
Dermatophytosis of scalp and beard (tinea capitis/ tinea barbae)
B352
Dermatophytosis of hand (tinea manuum)
B356
Dermatophytosis of groin and perianal area (tinea cruris)
B353
Dermatophytosis of foot (tinea pedis)
B355
Dermatophytosis of body (tinea corporis / tinea imbricate)
B358
Deep seated dermatophytosis
B358-B359
Dermatophytosis of other specified sites - unspecified site
B361
Tinea nigra
B370,B3783
Candidiasis of mouth
B3742,B3749
Candidiasis of other urogenital sites

Oregon Medicaid PA Criteria 22 July 1, 2017
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis funded by OHP? (See
examples in Table 1).
Yes: Go to #3
No: Go to #4
3. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require PA.
Preferred products are evidence-based
reviewed for comparative effectiveness
and safety.
Yes: Inform prescriber of
preferred alternatives.
No: Approve for 3
months or course of
treatment.
4. Is the prescriber a hematology, oncology
or infectious disease specialty prescriber
requesting voriconazole?
Yes: Approve for 3
months or course of
treatment.
No: Go to #5
5. Is the diagnosis not funded by OHP?
(see examples in Table 2).
Yes: Pass to RPh.
Deny; not funded by OHP
No: Got to #6
6. Is the diagnosis funded by OHP if criteria
are met?
(see examples in Table 3).
Yes: Go to #7
No: Go to #9
7. Is the patient immunocompromised
(examples below)?
Does the patient have a current (not
history of) diagnosis of cancer AND
is currently undergoing
Chemotherapy or Radiation?
Document therapy and length of
treatment. OR
Does the patient have a diagnosis
of HIV/AIDS? OR
Does the patient have sickle cell
anemia?
Poor nutrition, elderly or chronically
ill?
Other conditions as determined and
documented by a RPh.
Yes: Record ICD-10
code. Approve as follows:
(immunocompromised
patient)
ORAL & TOPICAL
Course of treatment.
If length of therapy is
unknown, approve
for 3 months.
No: Go to #8

Oregon Medicaid PA Criteria 23 July 1, 2017
Approval Criteria
8. Is the patient currently taking an
immunosuppressive drug? Document
drug.
Pass to RPh for evaluation if drug not in
list.
Immunosuppressive drugs include but are
not limited to:
azathioprine
leflunomide
basiliximab
mercaptopurine
cyclophosphamide
methotrexate
cyclosporine
mycophenolate
etanercept
rituximab
everolimus
sirolimus
hydroxychloroquine
tacrolimus
infliximab
Yes: Approve as follows:
(immunocompromised
patient)
ORAL & TOPICAL
Course of treatment.
If length of therapy is
unknown, approve for
3 months.
No: Pass to RPh. Deny;
not funded by the OHP
9. RPh only: All other indications need to be evaluated to see if it is an OHP-funded diagnosis:
If funded: may approve for treatment course with PRN renewals. If length of therapy is unknown,
approve for 3-month intervals only.
If not funded: Deny; not funded by the OHP.
o Deny non-fungal diagnosis (medical appropriateness)
o Deny fungal ICD-10 codes that do not appear on the OHP list pending a more specific
diagnosis code (not funded by the OHP).
o Forward any fungal ICD-10 codes not found in the Tables 1, 2, or 3 to the Lead Pharmacist.
These codes will be forwarded to DMAP to be added to the Tables for future requests.
P&T Review: 7/15 (kk); 09/10; 2/06; 11/05; 9/05; 5/05
Implemented: 5/1/16; 8/15; 1/1/11; 7/1/06; 11/1/0; 9/1/0
Oregon Medicaid PA Criteria 24 July 1, 2017
Antihistamines
Goals:
Approve antihistamines only for conditions funded by the OHP.
Allergic rhinitis treatment is covered by the OHP only when complicated by other diagnoses
(e.g. asthma, sleep apnea).
Promote use that is consistent with Oregon Asthma Guidelines and medical evidence.
http://public.health.oregon.gov/DiseasesConditions/ChronicDisease/Asthma/Pages/index.aspx
Length of Authorization:
6 months
Requires PA:
Non-preferred oral antihistamines and combinations
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require a PA.
Preferred products are evidence-based
reviewed for comparative effectiveness
and safety by the Oregon Pharmacy &
Therapeutics Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Go to #3
3. Does patient have a diagnosis of allergic
rhinitis, allergic conjunctivitis, or chronic
rhinitis/pharyngitis/nasopharyngitis?
Yes: Go to #4
No: Go to #8
4. Does the patient have asthma or reactive
airway disease exacerbated by
chronic/allergic rhinitis or allergies?
Yes: Go to #5
No: Go to #6

Oregon Medicaid PA Criteria 25 July 1, 2017
Approval Criteria
5. Does the drug profile show an asthma
controller medication (e.g. ORAL inhaled
corticosteroid, leukotriene antagonist, etc.)
and/or inhaled rescue beta-agonist (e.g.
albuterol) within the last 6 months?
Keep in mind: albuterol may not need to be
used as often if asthma is controlled on
other medications.
Yes: Approve for 6
months
No: Pass to RPh.
Deny; medical
appropriateness.
Oregon Asthma
guidelines recommend
all asthma clients have
access to rescue
inhalers and those with
persistent disease
should use anti-
inflammatory medicines
daily (preferably orally
inhaled corticosteroids).
6. Does patient have other co-morbid
conditions or complications that are funded?
Acute or chronic inflammation of the orbit
Chronic Sinusitis
Acute Sinusitis
Sleep apnea
Wegener’s Granulomatosis
Yes: Document ICD-10
codes. Go to #7
No: Pass to RPh.
Deny; not funded by the
OHP
7. Does patient have contraindications (e.g.
pregnancy), or had insufficient response to
available alternatives? Document.
Yes: Approve for up to 6
months
No: Pass to RPh. Deny;
medical
appropriateness
8. Is the diagnosis COPD or Obstructive
Chronic Bronchitis?
Yes: Pass to RPh.
Deny; medical
appropriateness.
Antihistamine not
indicated.
No: Go to #9
9. Is the diagnosis Chronic Bronchitis?
Yes: Pass to RPh.
Deny; not funded by the
OHP
No: Pass to RPh. Go to
#10
10. RPh only: Is the diagnosis above the line or below the line?
Above: Deny; medical appropriateness
Below: Deny; not funded by the OHP (e.g., acute upper respiratory infections or urticaria).
P&T Review: 5/15 (AG); 9/10; 9/08; 2/06; 9/04; 5/04; 2/02
Implementation: 5/1/16; 7/15, 1/11, 7/09, 7/06, 3/06, 10/04, 8/02, 9/06

Oregon Medicaid PA Criteria 26 July 1, 2017
Antimigraine - Triptans
Goal(s):
Decrease potential for medication overuse headache through quantity limits and therapeutic
duplication denials.
Promote PDL options.
Length of Authorization:
Up to 6 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Check the Reason for PA:
Non-Preferred drugs will deny on initiation
Preferred drugs will deny only when maximum dose exceeded
Both will deny for concurrent therapy (concurrent triptans by different routes is allowed)
Quantity Limits per Labeling.
Generic
Brand
Max Daily
Dose
Dosage Form
Quantity Limit Per
Month
Almotriptan
Axert
25 mg
6.25 mg tab
12.5 mg tab
12 tabs
Eletriptan
Relpax
80 mg
20 mg tab
40 mg tab
(blister pack 6, 12)
9 tabs
Frovatriptan
Frova
7.5 mg
2.5 mg tab
(blister pack 9)
9 tabs
Naratriptan
Amerge
5 mg
1 mg tab
2.5 mg tab (blister pack 9)
9 tabs
Rizatriptan
Maxalt
Maxalt MLT
30 mg
5 mg tab
10 mg tab (blister pack 6,
12)
12 tabs
Sumatriptan
tablets
Imitrex &
generics
200 mg
25 mg tab, 50 mg tab,
100 mg tab (blister pack 9)
9 tablets
Sumatriptan
nasal spray
Imitrex &
generics
40 mg
5 mg, 10 mg (box of 6)
18 spray units
Sumatriptan
nasal powder
Onzetra
Xsail
44 mg
22 mg (11 mg in each
nostril)
6 nosepieces
Sumatriptan
injectable
Imitrex &
generics
12 mg
6 mg/0.5 mL
6 vials

Oregon Medicaid PA Criteria 27 July 1, 2017
Generic
Brand
Max Daily
Dose
Dosage Form
Quantity Limit Per
Month
Sumatriptan
injectable
Sumavel
12 mg
6 mg/0.5 mL units
(package of 6)
6 jet injectors
Sumatriptan
injectable
Zembrace
Symtouch
12 mg
3 mg/0.5 mL
(package of 4)
12 auto-injectors
Sumatriptan
/naproxen
Treximet
170/1000 mg
(2 tablets)
85/500 mg tab
(box of 9)
9 tablets
Zolmitriptan
Zomig
Zomig ZMT
10 mg
2.5 mg tab
(blister pack, 6)
6 tabs
Zolmitriptan
nasal spray
Zomig NS
10 mg
5 mg (box of 6)
3 packages (18
spray units)
Abbreviations: d = days; MR = may repeat; NS = nasal spray; PO = orally
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Does the patient have a diagnosis
of migraine headaches?
Yes: Go to #3
No: Pass to RPh. Deny;
medical
appropriateness.
3. Is requested drug a preferred
product?
Yes: Go to #5
No: Go to #4
4. Will the prescriber consider a
change to a preferred product?
Message:
Preferred products do not
require PA within recommended
dose limits.
Preferred products are
evidence-based reviewed for
comparative effectiveness and
safety by the Oregon Pharmacy
& Therapeutics Committee.
Yes: Inform prescriber of
covered alternatives in class and
dose limits.
No: Go to #5

Oregon Medicaid PA Criteria 28 July 1, 2017
Approval Criteria
5. Is request for a higher dose than
listed in quantity limit chart?
Yes: Pass to RPh. Deny;
medical appropriateness.
May recommend use of
migraine prophylactic
therapy and reinforce that
doses above those
recommended by the
manufacturer increase the
incidence of medication
overuse headache.
One lifetime 90-day taper
may be approved at
pharmacist’s discretion.
Document.
No: Trouble-shoot claim
payment (e.g., days’
supply?).
Go to #6.
6. Is the request for two different oral
triptans concurrently?
Yes: Go to #7
No: Approve for 6
months
7. Is this a switch in Triptan therapy
due to intolerance, allergy or
ineffectiveness?
Yes: Document reason for switch
and override for concurrent use
for 30 days.
No: Pass to RPh. Deny;
medical
appropriateness.
P&T Review: 3/16 (MH); 3/10; 9/09; 11/03; 5/03
Implementation: 5/1/16, 3/23/10; 1/1/10; 7/1/06; 5/31/05; 6/30/04

Oregon Medicaid PA Criteria 29 July 1, 2017
Anti-Parkinson’s Agents
Goals:
Promote preferred drugs for Parkinson’s disease.
Restrict use for non-funded conditions like restless leg syndrome.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis Parkinson’s disease or
another chronic neurological condition?
Yes: Go to #5
No: Go to #3
3. Is the diagnosis Restless Leg Syndrome?
Yes: Pass to RPh.
Deny; not funded by the
OHP.
No: Go to #4
4. RPh only:
All other indications need to be evaluated to
determine if treatment is for a funded
condition.
Funded: Go to #5
Not Funded: Deny; not
funded by the OHP.
5. Will the prescriber consider a change to a
preferred product?
Message:
• Preferred products do not require PA.
• Preferred products are evidence-based
reviewed for comparative effectiveness &
safety by the Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Approve for the
shorter of 1 year or
length of prescription.
P&T Review: 7/16 (DE); 9/14; 9/13; 09/10
Implementation: 8/16, 1/1/14, 1/1/11

Oregon Medicaid PA Criteria 30 July 1, 2017
Antiplatelets
Goal:
Approve antiplatelet drugs for funded diagnoses which are supported by medical literature.
Length of Authorization:
Up to 12 months.
Requires PA:
Non-preferred drugs
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the diagnosis an OHP funded diagnosis?
Yes: Go to #3
No: Pass to RPh.
Deny, not funded by the
OHP.
3. Will the prescriber consider a change to a
preferred product?
Yes: Inform provider of
preferred alternatives.
No: Go to #4
4. Is this continuation of hospital treatment?
Yes: Approve for 30
days only and inform
provider of preferred
products.
No: Go to #5
5. Is the request for either prasugrel or
vorapaxar AND does the patient have a
history of stroke, TIA or intracranial
hemorrhage?
Yes: Deny for medical
appropriateness
No: Approve for FDA-
approved indications for
up to 1 year.
If vorapaxar is
requested, it should be
approved only when
used in combination
with aspirin and/or
clopidogrel. There is
limited experience with
other platelet inhibitor
drugs or as
monotherapy.

Oregon Medicaid PA Criteria 31 July 1, 2017
FDA Approved Indications (July 2015)
2
o
Stroke
2
o
PAD
2
o
MI
ACS
No PCI
PCI
ASA/DP ER
x
clopidogrel
x
x
x
x
x
prasugrel
CI
x
ticagrelor
x
x
vorapaxar
CI
x
x
Abbreviations: 2⁰ = secondary prevention; ACS=Acute Coronary Syndrome; ASA/DP ER = aspirin/dipyridamole;
CI=contraindication; PCI=Percutaneous Intervention; X = FDA-approved indication.
P&T / DUR Review: 7/15 (KK); 11/11
Implementation: 10/15, 8/15; 7/31/14; 4/9/12
Oregon Medicaid PA Criteria 32 July 1, 2017
Antivirals for Herpes Simplex Virus
Goal(s):
Cover oral and/or topical antivirals only for covered diagnoses.
HSV infections are covered only when complicated by an immunocompromised host.
Length of Authorization:
Up to 12 months (criteria specific)
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require a PA.
Preferred products are evidence-based
reviewed for comparative effectiveness
and safety by the Oregon Pharmacy &
Therapeutics Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Go to #3
3. Is the diagnosis uncomplicated herpes
simplex virus infection (B002; B0089; B001;
B009)?
Yes: Go to #4
No: Go to #7
4. Pass to RPh: Is the patient
immunocompromised (document ICD10
code).
Examples:
Diagnosis of cancer AND currently
undergoing chemotherapy or
radiation. Document therapy and
length of treatment.
Solid organ transplant
HIV/AIDS
Yes: Approve for up to
12 months
No: Go to #5

Oregon Medicaid PA Criteria 33 July 1, 2017
Approval Criteria
5. Is the patient currently taking an
immunosuppressive drug?
Document name of drug. If is drug not in the
list below, pass to RPh for evaluation.
Immunosuppressive drugs include, but are
not limited to:
Immunosuppressants
Abatacept
Adalimumab
Anakinra
Apremilast
Azathioprine
Basiliximab
Certolizumab pegol
Cyclosporine
Cyclosporine
Etanercept
Golimumab
Hydroxychloroquine
Infliximab
Leflunomide
Methotrexate
Natalizumab
Rituximab
Secukinumab
Sirolimus
Tacrolimus
Tocilizumab
Tofacitinib
Ustekinumab
Vedolizumab
Yes: Approve for up to
90 days
No: Pass to RPh. Go to
#6.
6. RPh only:
All other indications need to be evaluated as
to whether they are an OHP-funded
condition.
If funded and clinic
provides supporting
literature, approve for
length of treatment. If
length of treatment is not
provided, approve for 3
months.
Note: deny non-viral
diagnoses (medical
appropriateness)
If non-funded, deny (not
funded by the OHP).
Note: Deny viral ICD-10
codes that do not
appear on the OHP
funding list pending a
more specific diagnosis
code (not funded by the
OHP).
P&T Review: 7/16 (KS); 1/14; 1/12; 9/10 (KS)
Implementation: 8/16; 1/1/11

Oregon Medicaid PA Criteria 34 July 1, 2017
Antivirals - Influenza
Goal:
Restrict use of extended prophylactic influenza antiviral therapy to high risk populations
recognized by the Centers for Disease Control and Prevention (CDC) and Infectious Diseases
Society of America (IDSA).
Length of Authorization:
Up to 30 days
Requires PA:
Non-preferred neuraminidase inhibitors
Oseltamivir therapy for greater than 5 days
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is this an OHP-funded diagnosis?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP
3. Is the antiviral agent to be used to treat a
current influenza infection (ICD10 J1100,
J129, J111-112, J1181, J1189; J09X1-
J09X9)?
Yes: Go to #4
No: Go to #5
4. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require PA
Preferred products are evidence-based
reviewed for comparative effectiveness
and safety by the Oregon Pharmacy &
Therapeutics Committee.
Yes: Inform prescriber
of covered alternatives
in class and approve for
length of therapy or 5
days, whichever is less.
No: Approve for length
of therapy or 5 days,
whichever is less.
5. Is the antiviral prescribed oseltamivir or
zanamivir?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness.

Oregon Medicaid PA Criteria 35 July 1, 2017
Approval Criteria
6. Does the patient have any of the following
CDC
1
and IDSA
2
criteria that may place
them at increased risk for complications
requiring chemoprophylaxis?
Persons at high risk of influenza
complications during the first 2 weeks
following vaccination after exposure
to an infectious person (6 weeks in
children not previously vaccinated
and require 2 doses of vaccine)
Persons with severe immune
deficiencies or others who might not
respond to influenza vaccination,
such as persons receiving
immunosuppressive medications,
after exposure to an infectious person
Persons at high risk for complications
from influenza who cannot receive
influenza vaccine after exposure to
an infectious person
Residents of institutions, such as
long-term care facilities, during
influenza outbreaks in the institution.
Pregnancy and women up to 2 weeks
postpartum who have been in close
contact with someone suspected or
confirmed of having influenza
Yes: Approve for
duration of prophylaxis
or 30 days, whichever is
less.
Current recommended
duration of prophylaxis:
7 days (after last known
exposure; minimum 2
weeks to control
outbreaks in institutional
settings and hospitals,
and continue up to 1
week after last known
exposure.
.
No: Pass to RPh. Deny;
medical
appropriateness.
References:
1. Centers for Disease Control and Prevention. Influenza Antiviral Medications: Summary for Clinicians.
http://www.cdc.gov/flu/pdf/professionals/antivirals/antiviral-summary-clinician.pdf. Accessed June 2, 2015.
2. Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children – diagnosis, treatment, chemoprophylaxis, and
institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clinical Infectious
Diseases. 2009; 48:1003-32.
P&T/DUR Review: 1/16 (AG); 1/12; 9/10
Implementation: 10/13/16; 2/12/16; 1/11

Oregon Medicaid PA Criteria 36 July 1, 2017
Becaplermin (Regranex
®
)
Goal(s):
Restrict to indications funded by the OHP and supported by medical literature.
Length of Authorization:
Up to 6 months
Requires PA:
Becaplermin topical gel (Regranex
®
)
Covered Alternatives:
No preferred alternatives
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Does the patient have an ulcer(s) (ICD10
E0842; E0942; E1042; E1142; E1342;
L97109; L97209; L97309; L97409; L97509;
L97809; L98419; L98429; L98499)?
Yes: Go to #3.
No: Pass to RPh.
Deny; medical
appropriateness.
3. Does the patient have diabetes mellitus?
Yes: Approve ONLY 15
grams for 6-month
supply.
No: Pass to RPh.
Deny; medical
appropriateness.
P&T/DUR Review: 09/15 (AG)
Implementation: 10/15
Oregon Medicaid PA Criteria 37 July 1, 2017
Benign Prostatic Hypertrophy (BPH) Medications
Goal(s):
BPH with urinary obstruction is an OHP-funded treatment only when post-void residuals are
150 mL or more.
Restrict use for male pattern baldness and erectile dysfunction, which are not OHP-funded
conditions.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Will the prescriber consider switching to a
preferred product?
Message:
Preferred products do not require a PA.
Preferred products are evidence-based
reviewed for comparative effectiveness
and safety by the Oregon Pharmacy &
Therapeutics Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Go to #3
3. Is the request for continuation of therapy
previously approved by the FFS program?
Yes: Go to Renewal
Criteria
No: Go to #4
4. Is the request for an alpha-1 blocker, and
does the patient have a diagnosis related to
functional and mechanical disorders of the
genitourinary system including bladder
outlet obstruction?
Yes: Go to #5
No: Go to #6
5. Has the patient tried and failed a 2-month
trial of a preferred alpha-1 blocker?
Yes: Approve an alpha-
1 blocker for up to 12
months
No: Pass to RPh. Deny
until patient has tried
and failed a covered
alternative
6. Does the patient have a diagnosis of benign
prostatic hypertrophy (BPH) or enlarged
prostate with obstruction?
Yes: Approve for up to
12 months
No: Go to #7

Oregon Medicaid PA Criteria 38 July 1, 2017
Approval Criteria
7. Does the patient have a diagnosis of
unspecified urinary obstruction or BPH
without obstruction?
Yes: Pass to RPh.
Deny; not funded by the
OHP
No: Pass to RPh. Go to
#8
8. RPh Only: All other conditions need to be evaluated to see if diagnosis is funded:
Funded: covered diagnoses related to prostate may be approved for 1 year.
Not Funded: unfunded diagnoses (e.g., hair growth, erectile dysfunction) should be denied (not
funded by the OHP).
Alpha-1 blockers and 5-alpha reductase inhibitors may be used concurrently for BPH up to
1 year. Alpha-1 blockers may be discontinued once prostate is reduced to normal size.
If urine retention (obstructive), ask for more specific diagnosis.
Renewal Criteria
1. Is the request for an alpha-1 blocker and
does the patient have a diagnosis related to
functional and mechanical disorders of the
genitourinary system including bladder outlet
obstruction?
Yes: Go to #2
No: Go to #3
2. Has the patient also been taking a 5-alpha
reductase inhibitor for the last year?
Yes: Recommend
against combination
therapy exceeding 1
year.
No: Approve for the
shorter of 12 months or
length of the
prescription
3. Does the patient have a diagnosis of BPH or
enlarged prostate with obstruction?
Yes: Approve for up to
12 months
No: Go to #4
4. Does the patient have a diagnosis of
unspecified urinary obstruction or benign
prostatic hyperplasia without obstruction?
Yes: Pass to RPh. Deny;
not funded by the OHP
No: Pass to RPh. Go to
#5
5. RPh only:
All other indications need to be evaluated as to
whether they are a funded condition:
Alpha Blockers and 5-alpha
reductase inhibitors may be used
concurrently for BPH up to 1 year.
Alpha-blockers may be discontinued
once prostate is reduced to normal
size.
If urine retention, obstructive, ask for
more specific diagnosis.
If funded and clinic
provides supporting
literature, approve for up
to 12 months.
If non-funded, deny (not
funded by the OHP).
P&T Review: 7/16 (KS); 11/12; 9/10; 3/10; 5/08; 2/06
Implementation: 8/16, 2/21/13; 1/1/11; 4/20/10; 5/22/08; 7/1/06; 9/30/05

Oregon Medicaid PA Criteria 39 July 1, 2017
Benzodiazepines
Goal(s):
Approve only for OHP-funded diagnoses.
Prevent inappropriate long-term benzodiazepine use beyond 4 weeks for new starts (no history
within the last 120 days).
Approve long-term use only for indications supported by the medical literature.
Length of Authorization:
6 months to 12 months (criteria-specific)
Requires PA:
All benzodiazepines used beyond 4 weeks. Short-term use does not require PA.
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Does the patient have a malignant
neoplasm or other end-of-life diagnosis
(ICD10 C00.xx-D49.xx or Z51.5)?
Yes: Approve for 12
months
No: Go to #3
3. Does the patient have a seizure disorder
diagnosis (ICD10 G40.xx; F44.5; R56.9;
G93.81; R56.1; R56.9; G93.81; G83.8;
P90)?
Yes: Approve for 12
months
No: Go to #4
4. Is the diagnosis an OHP-funded diagnosis?
Yes: go to #5
No: Pass to RPh. Deny;
not funded by the OHP.
5. Is the patient on a concurrent sedative,
hypnotic or opioid?
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Go to #6
6. RPh only: is there appropriate rationale to
support long-term benzodiazepine use for
this indication?
Yes: Approve for up to 6
months.
No: Deny; medical
appropriateness.
P&T Review: 3/27/2014
Implementation: 5/1/16

Oregon Medicaid PA Criteria 40 July 1, 2017
Biologics for Autoimmune Diseases
Goal(s):
Restrict use of biologics to OHP funded conditions and according to OHP guidelines for use.
Promote use that is consistent with national clinical practice guidelines and medical evidence.
Promote use of high value products.
Length of Authorization:
Up to 12 months
Requires PA:
All biologics except for biologics approved by the FDA for the following indications:
o Non-Hodgkin Lymphoma (ICD-10 C85.8x, C85.9x)
o Chronic Lymphocytic Leukemia (ICD-10 C91.10, C91.11, C91.12)
o Juvenile Idiopathic Arthritis (ICD-10 M08)
o Multiple Sclerosis (ICD-10 G35)
o Non-infectious posterior uveitis (ICD-10 H44.13)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Table 1. Approved Indications for Biologic Immunosuppressants.
Drug Name
Ankylosing
Spondylitis
Crohn’s
Disease
Juvenile
Idiopathic
Arthritis
Plaque
Psoriasis
Psoriatic
Arthritis
Rheumatoid
Arthritis
Ulcerative
Colitis
Uveitis
(non-
infec-
tious)
Other
Abatacept
(ORENCIA)
≥6 yo
≥18 yo
Adalimumab
(HUMIRA)
≥18 yo
≥6 yo
≥2 yo
≥18 yo
≥18 yo
≥18 yo
≥18 yo
≥18 yo
Alefacept
(AMEVIVE)
≥18 yo
Anakinra
(KINERET)
≥18 yo
NOMID
Apremilast
(OTEZLA)
≥18 yo
≥18 yo
Canakinumab
(ILARIS)
≥2 yo
FCAS ≥4 yo
MWS ≥4 yo
Certolizumab
(CIMZIA)
≥18 yo
≥18 yo
≥18 yo
≥18 yo
Etanercept
(ENBREL)
≥18 yo
≥2 yo
≥18 yo
≥18 yo
≥18 yo
Golimumab
(SIMPONI)
≥18 yo
≥18 yo
≥18 yo
≥18 yo
Infliximab
(REMICADE)
≥18 yo
≥6 yo
≥18 yo
≥18 yo
≥18 yo
≥6 yo
Ixekizumab
(TALTZ)
≥18 yo
Natalizumab
(TYSABRI)
≥18 yo
MS ≥18 yo
Rituximab
(RITUXAN)
≥18 yo
CLL ≥18 yo
NHL ≥18 yo
GPA ≥18 yo
Secukinumab
≥18 yo
≥18 yo
≥18 yo

Oregon Medicaid PA Criteria 41 July 1, 2017
(COSENTYX)
Tocilizumab
(ACTEMRA)
≥2 yo
≥18 yo
Tofacitinib
(XELJANZ)
≥18 yo
Ustekinumab
(STELARA)
≥18 yo
≥18 yo
Vedolizumab
(ENTYVIO)
≥18 yo
≥18 yo
Abbreviations: CLL = chronic lymphocytic leukemia; FCAS = familial cold autoinflammatory syndrome; GPA = granulomatosis with
polyangiitis (Wegener’s granulomatosis); MS = multiple sclerosis; MWS = Muckle-Wells syndrome; NHL = non-Hodgkin’s lymphoma;
NOMID = neonatal onset multi-systemic inflammatory disease; yo = years old.
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the diagnosis funded by OHP?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP.
3. Will the prescriber change to a preferred
product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
Committee.
Yes: Inform prescriber
of preferred alternatives.
No: Go to #4
4. Is the prescription for rituximab for non-
Hodgkin Lymphoma (ICD-10 C85.8x;
C85.9x) or Chronic Lymphocytic Leukemia
(ICD-10 C91.10; C91.11; C91.12)?
Yes: Approve for length
of treatment.
No: Go to #5
5. Is the prescription for natalizumab,
prescribed for the management of relapsing
multiple sclerosis?
Yes: Approve for length
of treatment.
No: Go to #6
6. Is the diagnosis juvenile idiopathic arthritis
(ICD-10 M08), non-infectious posterior
uveitis, or ankylosing spondylitis (ICD-10
M45) and the request for a drug FDA-
approved for one of these conditions as
defined in Table 1?
Yes: Approve for length
of treatment.
No: Go to #7
7. Is the diagnosis plaque psoriasis and the
request for a drug FDA-approved for this
condition as defined in Table 1?
Note: Only treatment for severe plaque
psoriasis is funded by the OHP.
Yes: Go to #8
No: Go to #10

Oregon Medicaid PA Criteria 42 July 1, 2017
Approval Criteria
8. Is the plaque psoriasis severe in nature,
which has resulted in functional impairment
(e.g., inability to use hands or feet for
activities of daily living, or significant facial
involvement preventing normal social
interaction) and one or more of the
following:
At least 10% body surface area
involvement; or
Hand, foot or mucous membrane
involvement?
Yes: Go to #9
No: Pass to RPh. Deny;
not funded by the OHP.
9. Has the patient failed to respond to each of
the following first-line treatments:
Topical high potency corticosteroid
(e.g., betamethasone dipropionate
0.05%, clobetasol propionate 0.05%,
fluocinonide 0.05%, halcinonide 0.1%,
halobetasol propionate 0.05%;
triamcinolone 0.5%); and
At least one other topical agent:
calcipotriene, tazarotene, anthralin; and
Phototherapy; and
At least one other systemic therapy:
acitretin, cyclosporine, or methotrexate?
Yes: Document each
therapy with dates: -
_________________
Approve for up to 12
months
No: Pass to RPh. Deny;
medical
appropriateness.
10. Is the diagnosis rheumatoid arthritis or
psoriatic arthritis and the request for a drug
FDA-approved for these conditions as
defined in Table 1?
Yes: Go to #11
No: Go to #14
11. Has the patient failed to respond to at least
one of the following disease-modifying
antirheumatic drugs (DMARD) for ≥6
months:
Methotrexate, leflunomide, or
sulfasalazine or hydroxychloroquine;
or
Have a documented intolerance or
contraindication to DMARDs?
Yes: Document each
therapy with dates: -
________________
If applicable, document
intolerance or
contraindication(s):
___________________
______
Go to #12
No: Pass to RPh. Deny;
medical
appropriateness.
12. Is the request for tofacitinib?
Yes: Go to #13
No: Approve for up to 12
months

Oregon Medicaid PA Criteria 43 July 1, 2017
Approval Criteria
13. Is the patient currently on other biologic
therapy or on a potent immunosuppressant
like azathioprine or cyclosporine?
Note: Tofacitinib may be used concurrently
with methotrexate or other oral DMARD
drugs.
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Approve for up to 12
months
14. Is the diagnosis Crohn’s disease or
ulcerative colitis and the request for a drug
FDA-approved for these conditions as
defined in Table 1?
Yes: Go to #15
No: Go to #16
15. Has the patient failed to respond to at least
one of the following conventional
immunosuppressive therapies for ≥6
months:
Mercaptopurine, azathioprine, or
budesonide; or
Have a documented intolerance or
contraindication to conventional
therapy?
Yes: Document each
therapy with dates: -
________________
If applicable, document
intolerance or
contraindication(s):
___________________
______
Approve for up to 12
months
No: Pass to RPh. Deny;
medical
appropriateness.
16. Is the diagnosis Granulomatosis with
Polyangiitis and the requested drug
rituximab for induction of remission?
Yes: Approve for length
of treatment
No: Go to #19
17. Is the diagnosis Granulomatosis with
Polyangiitis and the requested drug
rituximab for maintenance of remission?
Yes: Go to #18
No: Go to #19
18. Has the patient failed to respond to at least
one of the following conventional
immunosuppressive therapies for
maintenance of remission, in conjunction
with a low-dose corticosteroid, for ≥6
months:
Azathioprine, leflunomide, or
methotrexate
Have a documented intolerance or
contraindication to DMARDs?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness.
Oregon Medicaid PA Criteria 44 July 1, 2017
Approval Criteria
19. Is the diagnosis a variant cryopyrin-
associated periodic syndrome (Familial
Cold Auto-inflammatory Syndrome, Muckle-
Wells Syndrome, or chronic infantile
neurologic cutaneous articular syndrome
[also known as neonatal onset multi-
systemic inflammatory disease]) and the
request for a drug FDA-approved for one of
these conditions as defined in Table 1?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness.
P&T/DUR Review: 11/16 (AG); 9/16; 3/16; 7/15; 9/14; 8/12
Implementation: 1/1/17; 9/27/14; 2/21/13
Oregon Medicaid PA Criteria 45 July 1, 2017
Bone Resorption Inhibitors and Related Agents
Goal(s):
To ensure appropriate drug use and safety of bone resorption suppression agents by
authorizing utilization in specified patient populations.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is this an OHP-funded condition?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP
3. Will the prescriber consider a change to a
preferred product?
Note:
Preferred products do not require a PA.
Preferred products are evidence-based
reviewed for comparative effectiveness
and safety by the Oregon Pharmacy &
Therapeutics Committee
Yes: Inform prescriber
of covered alternatives
in class
No: Go to #4
4. Is the request for raloxifene?
Yes: Go to #5
No: Go to #6
5. Is the patient pregnant and/or at increased
risk for thromboembolism or stroke?
Yes: Pass to RPh.
Deny; medical
appropriateness.
Note: inform prescriber
of pregnancy category X
and boxed warning for
venous
thromboembolism and
stroke.
No: Approve for up to
12 months

Oregon Medicaid PA Criteria 46 July 1, 2017
Approval Criteria
6. Is the request for teriparatide and is the
patient at high risk for fractures?
Examples include:
Postmenopausal women with
osteoporosis
Men with primary or hypogonadal
osteoporosis
Osteoporosis associated with sustained
glucocorticoid therapy
Yes: Go to #7
No: Pass to RPh. Go to
#8
7. Does the patient meet one of the following
conditions:
a. Concomitant bisphosphonate; or
b. Pediatric or young adult with open
epiphyses; or
c. History of osteosarcoma or skeletal
malignancies; or
d. Metabolic bone disease; or
e. Underlying hypercalcemic disorders;
or
f. Unexplained elevated alkaline
phosphatase levels?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Approve for up to
12 months
8. RPh only:
All other indications need to be evaluated as
to whether they are funded by the OHP or
not.
If funded and clinic
provides supporting
literature, approve for up
to 12 months
If non-funded, deny; not
funded by the OHP
P&T Review: 7/16; 9/10
Implementation: 8/16, 1/1/11

Oregon Medicaid PA Criteria 47 July 1, 2017
Botulinum Toxins
Goal(s):
Approve botulinum toxins for funded OHP conditions supported by evidence of benefit (eg,
dystonia or spasticity associated with certain neurological diseases).
Require positive response to therapy for use in chronic migraine headaches or overactive bladder.
Length of Authorization:
From 90 days to 12 months
Requires PA:
Use of botulinum toxins without associated dystonia or neurological disease diagnosis in last 12
months.
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is this a request for renewal of a previously
approved prior authorization for
management of migraine headache or
detrusor over-activity (eg, overactive
bladder)?
Yes: Go to Renewal
Criteria
No: Go to #2
2. What diagnosis is being treated?
Record ICD10 code

Oregon Medicaid PA Criteria 48 July 1, 2017
Approval Criteria
3. Does patient have diagnosis of neurological-
induced dystonia or spasticity in which a
botulinum toxin is a first-line treatment
option?
Examples:
Genetic torsion dystonia (G241);
Acquired torsion dystonia (G803; G2402;
G248);
Blepharospasm (G245);
Spasmodic torticollis (G243);
Other fragments of torsion dystonia
(G249);
Paralysis associated with CVD (I69931-
I69969);
Multiple sclerosis (G35);
Neuromyelitis optica (G360);
Spastic hemiplegia, other specified
hemiplegia (G8100-G8194);
Cerebral palsy (G800-G809);
Quadriplegia and quadraparesis (-
G8250-G8254);
Paraplegia (G8220);
Diplegia of upper limbs (G830);
Monoplegia of lower limb (G8310-
G8314);
Monoplegia of upper limb (G8320-
G8324);
Unspecified monoplegia (G8330);
Other specified paralytic syndrome
(G8381-G8389);
Muscular dystrophies (G710-G712); or
Strabismus in other neuromuscular
disorders (H5089).
Yes: Approve for up to
12 months
No: Go to #4
4. Does patient have a diagnosis of chronic
migraine with ≥15 headache days per
month, of which ≥8 days are with migraine?
Yes: Go to #5
No: Go to #7
5. Is the botulinum toxin administered by, or in
consultation with, a neurologist or headache
specialist?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness.

Oregon Medicaid PA Criteria 49 July 1, 2017
Approval Criteria
6. Has the patient had an inadequate
response, or has contraindications, to ≥1
drugs from each of the following 3 drug
classes?
Beta-blockers: (propranolol; metoprolol;
atenolol; nadolol; or timolol)
Tricyclic antidepressants: (nortriptyline or
amitriptyline)
Anticonvulsants: (divalproex
sodium/valproic acid; carbamazepine;
topiramate; or gabapentin)
Calcium channel blockers (diltiazem;
verapamil; or nimodipine)
Yes:
Baseline
headaches/month:
_________.
Approve no more than 2
treatments given ≥3
months apart.
Additional treatment
requires documented
positive response to
therapy from baseline
(see Renewal Criteria).
No: Pass to RPh. Deny;
medical
appropriateness.
Recommend trial of
preferred alternatives at
www.orpdl.org/drugs/
7. Does patient have a diagnosis idiopathic or
neurogenic detrusor over-activity (eg,
overactive bladder syndrome) (ICD10-CM
N32.81)?
Yes: Go to #8
No: Pass to RPh. Go to
#9
8. Has the patient had an inadequate response
to, or is intolerant of, ≥2 incontinence anti-
muscarinic drugs (eg, fesoterodine,
oxybutynin, solifenacin, darifenacin,
tolterodine, or trospium)?
Yes:
Baseline urine
frequency/day:
_________.
Baseline urine
incontinence
episodes/day:
_________.
Approve for up to 90
days.
Additional treatment
requires documented
positive response to
therapy from baseline
(see Renewal Criteria).
No: Pass to RPh.
Deny; medical
appropriateness.

Oregon Medicaid PA Criteria 50 July 1, 2017
Approval Criteria
9. RPh only: Medical literature with evidence for use in funded conditions must be submitted and
determined to be appropriate for use before approval is granted.
Deny for the following conditions; not funded by the OHP
Neurologic conditions with none or minimally effective treatment or treatment not necessary
(G244; G2589; G2581; G2589; G259);
Facial nerve disorders (G510-G519);
Spastic dysphonia (J387);
Anal fissure (K602);
Disorders of sweat glands (eg, focal hyperhidrosis) (L301; L740-L759; R61);
Other disorders of cervical region (M436; M4802; M530; M531; M5382; M5402; M5412; M542;
M6788);
Acute and chronic disorders of the spine without neurologic impairment (M546; M545; M4327;
M4328; M532X7; M532X8; M533; M438X9; M539; M5408; M545; M5430; M5414-M5417;
M5489; M549);
Disorders of soft tissue (M5410; M609; M790-M792; M797);
Headaches (G44209; G44009; G44019; G44029; G44039; G44049; G44059; G44099; G44209;
G44219; G44221; G44229; G44309; G44319; G44329; G4441; G4451-G4453; G4459; G4481-
G4489; G441; R51);
Gastroparesis (K3184)
Deny for medical appropriateness for the following conditions; evidence of benefit is
insufficient
Dysphagia (R130; R1310-R1319);
Other extrapyramidal disease and abnormal movement disorders (G10; G230-GG238; G2401;
G244; G250-G26);
Other disorders of binocular eye movements (eg, esotropia, exotropia, mechanical strabismus,
etc.) (H4900-H518);
Tics (F950-F952; F959);
Laryngeal spasm (J385);
Spinal stenosis in cervical region or brachial neuritis or radiculitis NOS (M4802; M5412-M5413);
Spasm of muscle in absence of neurological diagnoses (M6240-M62838);
Contracture of tendon (sheath) in absence of neurological diagnoses (M6240; M62838);
Amyotrophic sclerosis (G1221);
Clinically significant spinal deformity or disorders of spine with neurological impairment (M4800;
M4804; M4806; M4808; M5414-M5417);
Hyperplasia of prostate (N400-N403; N4283)
Renewal Criteria
1. Is this a request for renewal of a previously
approved prior authorization for
management of migraine headache?
Yes: Go to #2
No: Go to #3

Oregon Medicaid PA Criteria 51 July 1, 2017
Renewal Criteria
2. Is there documentation of a reduction of ≥6
headache days per month compared to
baseline headache frequency?
Yes: Approve for up to
12 months
Baseline:____
headaches/month
Current:____
headaches/month
No: Pass to RPh. Deny;
medical appropriateness
3. Is this a request for renewal of a previously
approved prior authorization for
management of idiopathic or neurogenic
detrusor over-activity?
Yes: Go to #4
No: Go to Approval
Criteria
4. Is there a reduction of urinary frequency of
≥8 episodes per day or urinary incontinence
of ≥2 episodes per day compared to
baseline frequency?
Yes: Approve for up to
12 months
Baseline:____ urine
frequency/day
Current:____ urine
frequency/day
-or-
Baseline:____ urine
incontinence
episodes/day
Current:____ urine
incontinence
episodes/day
No: Pass to RPh. Deny;
medical appropriateness
P&T / DUR Review: 9/16 (AG); 11/15; 9/14; 7/14
Implementation : 10/13/16; 1/1/16

Oregon Medicaid PA Criteria 52 July 1, 2017
Buprenorphine and Buprenorphine/Naloxone
Goals:
Encourage use of buprenorphine products on the Preferred Drug List.
Restrict use of buprenorphine products under this PA to management of opioid use disorder.
Restrict use of oral transmucosal buprenorphine monotherapy products (without naloxone) to
pregnant patients or females actively trying to conceive.
Length of Authorization:
Up to 6 months
Requires PA:
Buprenorphine sublingual tablets
Suboxone
®
and generics (buprenorphine/naloxone) film and sublingual tablets that exceed an
average daily dose of 24 mg per day of buprenorphine
Bunavail
®
(buprenorphine/naloxone buccal film)
Zubsolv
®
(buprenorphine/naloxone sublingual tablets)
Probuphine
®
(buprenorphine subdermal implants)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the prescription for opioid use disorder
(opioid dependence or addiction)?
Yes: Go to #3
No: Pass to RPh. Deny;
medical appropriateness
3. Is the patient part of a comprehensive
treatment program for substance abuse
that includes psychosocial support
system(s)?
Yes: Go to #4
No: Pass to RPh. Deny;
medical appropriateness.
Buprenorphine therapy
must be part of a
comprehensive treatment
program that includes
psychosocial support.
4. Is the prescriber enrolled in the Oregon
Prescription Drug Monitoring Program
(www.orpdmp.com) and has the
prescriber verified at least once in the
past 6 months that the patient has not
been prescribed any opioid analgesics
from other prescribers?
Yes: Go to #5
No: Pass to RPh. Deny;
medical appropriateness
5. Is the requested medication a preferred
agent?
Yes: Go to #7
No: Go to #6

Oregon Medicaid PA Criteria 53 July 1, 2017
Approval Criteria
6. Will the prescriber switch to a preferred
product?
Note: Preferred products are reviewed for
comparative safety and efficacy by the
Oregon Pharmacy and Therapeutics
Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Go to #7
7. Is the request for the buprenorphine
implant system (Probuphine)?
Yes: Go to #8
No: Go to #9
8. Has the patient been clinically stable on 8
mg daily or less of Suboxone or Subutex
(or equivalent, see Table 1) for at least 6
months?
Note: see Table 1 for definition of clinical
stability and for equivalent dosing of other
buprenorphine products.
Yes: if all criteria in
Table 1 met, approve 4
implants for 6 months
No: Pass to RPh. Deny;
medical appropriateness
9. Is the prescription for a transmucosal
formulation of buprenorphine (film, tablet)
with an average daily dose of more than
24 mg (e.g., >24 mg/day or >48 mg every
other day)?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #10
10. Is the prescribed product a buprenorphine
monotherapy product (i.e., without
naloxone)
Yes: Go to #11
No: Go to #13
11. Is the patient pregnant or a female
actively trying to conceive?
Yes: Go to #13
No: Go to #12
12. Does the patient have a contraindication
or intolerance to buprenorphine/naloxone
combination products that prevents
successful management of opioid use
disorder?
Yes: Go to #13
No: Pass to RPh. Deny;
medical appropriateness
13. What is the expected length of treatment?
Document length of therapy: ____________
Approve for anticipated length of treatment or 6
months, whichever is shorter.
Table 1. Criteria for Approved Use of Probuphine (buprenorphine implant).
1
PROBUPHINE implants are only for use in patients who meet ALL of the following criteria:
Patients should not be tapered to a lower dose for the sole purpose of transitioning to PROBUPHINE
Stable transmucosal buprenorphine dose (of 8 mg per day or less of a sublingual Subutex or Suboxone sublingual
tablet or its transmucosal buprenorphine product equivalent) for 3 months or longer without any need for supplemental
dosing or adjustments:

Oregon Medicaid PA Criteria 54 July 1, 2017
o Examples of acceptable daily doses of transmucosal buprenorphine include:
Subutex (buprenorphine) sublingual tablet (generic equivalent) 8 mg or less
Suboxone (buprenorphine and naloxone) sublingual tablet (generic equivalent) 8 mg/2 mg or less
Bunavail (buprenorphine and naloxone) buccal film 4.2 mg/0.7 mg or less
Zubsolv (buprenorphine and naloxone) sublingual tablets 5.7 mg/1.4 mg or less
Consider the following factors in determining clinical stability and suitability for PROBUPHINE treatment:
no reported illicit opioid use
low to no desire/need to use illicit opioids
no reports of significant withdrawal symptoms
stable living environment
participation in a structured activity/job that contributes to the community
consistent participation in recommended cognitive behavioral therapy/peer support program
stability of living environment
participation in a structured activity/job
Reference: PROBUPHINE (buprenorphine implant for subdermal administration) [Prescribing Information]. Princeton, MJ:
Braeburn Pharmaceuticals, Inc., May 2016.
P&T/DUR Review: 1/17 (AG); 9/16; 1/15; 9/09; 5/09
Implementation: 4/1/2017; 9/1/13; 1/1/10

Oregon Medicaid PA Criteria 55 July 1, 2017
Calcium and Vitamin D Supplements
Goal(s):
Restrict use of calcium and vitamin D supplements to patients who are pregnant; have a
documented nutritional deficiency; have a diagnosis of osteopenia or osteoporosis; or elderly
patients at risk for falls.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred calcium and vitamin D products
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is this an OHP-funded diagnosis?
Yes: Go to #3
No: Pass to RPh. Deny; not
funded by the OHP
3. Does the patient meet any of the following
criteria:
Pregnancy;
Documented nutrient deficiency;
Diagnosis of osteopenia or
osteoporosis;
OR
Age 65 years or older and at risk for
falls
Yes: Approve for
up to 12 months.
Request that a 90
day’s supply be
filled at a time.
No: Pass to RPh. Deny;
medical appropriateness
P&T Review: 3/16 (KS)
Implementation: 5/1/16
Oregon Medicaid PA Criteria 56 July 1, 2017
Clobazam
Goal(s):
To ensure appropriate drug use and restrict to indications supported by medical literature.
Length of Authorization:
12 months
Requires PA:
Clobazam
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Does the patient have a diagnosis of
Lennox-Gastaut syndrome and is 2 years of
age or older?
Yes: Go to #3
No: Pass to RPh. Deny;
medical
appropriateness
3. Is the patient uncontrolled on current
baseline therapy with at least one other
antiepileptic medication?
Yes: Approve for 12
months
No: Pass to RPh. Deny;
medical
appropriateness
Limitations of Use:
• Clobazam is not indicated for other epilepsy syndromes other than Lennox-Gastaut.
P&T Review: 7/16 (DM); 3/15; 5/12
Implementation: 8/16, 8/12

Oregon Medicaid PA Criteria 57 July 1, 2017
Codeine
Goal(s):
Promote safe use of codeine in pediatric patients for analgesia or cough.
Length of Authorization:
Up to 3 days
Requires PA:
All codeine products for patients under 19 years of age
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. What is the age of the patient?
Ages 0-12 years: Pass
to RPh. Deny; medical
appropriateness
Ages 13-18 years: Go
to #3
3. Is the prescription for an OHP-funded
condition?
Yes: Go to #4
No: Pass to RPh. Deny;
not funded by the OHP
4. Has the patient recently undergone
tonsillectomy or adenoidectomy?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #5
5. Does the dose exceed 240 mg per day?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Approve no more
than 3-day supply
P&T Review: 5/16; 9/15; 7/15
Implementation: 7/1/16; 8/25/15

Oregon Medicaid PA Criteria 58 July 1, 2017
Conjugated Estrogens/Bazedoxifene (Duavee
®
)
Goal(s):
Approve conjugated estrogens/bazedoxifene only for indications where there is evidence to
support its use and safety.
Support the use of agents with clinical efficacy and safety supported by the medical literature and
guidelines.
Initiative:
Prior Authorization
Length of Authorization:
6-12 months
Requires PA:
Conjugated estrogens/bazedoxifene
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Step Therapy Required Prior to Coverage:
Prevention of vasomotor symptoms: conventional hormone therapy (see preferred drug list
options at (www.orpdl.org)
Prevention of osteoporosis: bisphosphonates (see preferred drug list options at
www.orpdl.org).
Approval Criteria
1. What is the diagnosis?
Record ICD10 code
2. Is patient a postmenopausal woman within
10 years of menopause?
Yes: Go to #3
No: Pass to RPh. Deny;
medical
appropriateness.
3. Is the patient <60 years of age with an intact
uterus?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require a co-
pay. Preferred products are evidence-
based reviewed for comparative
effectiveness and safety by the Oregon
Pharmacy & Therapeutics (P&T)
Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Go to #5

Oregon Medicaid PA Criteria 59 July 1, 2017
Approval Criteria
5. Is the patient being prescribed the
medication for the prevention of
osteoporosis?
Yes: Go to #6
No: Go to #7
6. Has the patient tried and failed, or is there a
contraindication to, bisphosphonates?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness
7. Is the medication being prescribed for the
prevention of vasomotor symptoms?
Yes: Go to #8
No: Pass to RPh. Deny;
medical
appropriateness
8. Has the patient tried and failed or has a
contraindication to conventional hormone
therapy?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness
P&T Review: 1/17 (SS), 11/14
Implementation: 4/1/17; 1/1/15

Oregon Medicaid PA Criteria 60 July 1, 2017
Cough and Cold Preparations
Goal(s):
Limit use of cough and cold preparations to OHP-funded diagnoses.
Symptomatic treatment of upper respiratory tract infections is not funded by the OHP.
Length of Authorization:
Up to 12 months
Requires PA:
All drugs (expectorants, antitussives, oral decongestants and combinations) in TC = 16, 17
except those listed below.
All products for patients under 13 years of age.
All codeine-containing products for patients under 19 years of age (see Codeine PA criteria).
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
HSN
Generic Drug Name
000206
Guaifenesin/codeine
000223
Guaifenesin/Dextromethorphan
002091
Pseudoephedrine
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the diagnosis an OHP-funded diagnosis?
All indications need to be evaluated to see if
funded on the Oregon Health Plan list of
prioritized services.
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP.
3. Has the patient tried and failed, or have
contraindications to, one of the covered
alternatives listed above?
Yes: document failure.
Approve for up to 1 year.
No: Pass to RPh. Deny;
cost-effectiveness
P&T Review: 5/16 (KK); 5/13; 2/06
Implementation: 7/1/16; 1/10/08
Oregon Medicaid PA Criteria 61 July 1, 2017
Cysteamine Delayed-release (PROCYSBI
®
)
Goal(s):
To restrict use of costly agents to appropriate patient populations.
Length of Authorization:
Up to 6 months
Requires PA:
Cysteamine delayed-release capsules (PROCYSBI)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis nephropathic cystinosis?
Yes: Go to #3
No: Pass to RPh. Deny;
medical
appropriateness.
3. Is the patient receiving medications through
a gastrostomy tube?
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Go to #4
4. Has the patient had an adequate trial of
cysteamine immediate-release (IR) capsules
(CYSTAGON); AND
Is the prescriber experienced in managing
metabolic diseases such as nephropathic
cystinosis; AND
Is there documentation of justified patient
non-adherence to cysteamine IR that
prevents the patient from achieving WBC
cysteine levels (<1 nmol ½ cysteine per mg
protein)?
Yes: Approve for up to 6
months.
No: Pass to RPh. Deny;
medical
appropriateness.
P&T/DUR Review: 11/16 (DM); 3/14
Implementation: 1/1/17; 5/1/14

Oregon Medicaid PA Criteria 62 July 1, 2017
Daclizumab (Zinbryta™)
Goal(s):
Restrict use of daclizumab to patients with relapsing multiple sclerosis (RMS) who have failed
multiple drugs for the treatment of RMS.
Ensure appropriate baseline monitoring to minimize patient harm.
Length of Authorization:
6 months
Requires PA:
Zinbryta™ (daclizumab)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the patient an adult (age ≥18 years)
diagnosed with relapsing multiple
sclerosis (RMS)?
Yes: Go to #3
No: Pass to RPh.
Deny; medical
appropriateness
3. Has the patient failed trials for at least 2
drugs indicated for the treatment of
RMS?
Yes: Document drug and
dates trialed:
1._________________(dates)
2._________________(dates)
(3.)________________(dates)
(4.)________________(dates)
Go to #4
No: Pass to RPh.
Deny; medical
appropriateness
4. Does the patient have a higher degree of
ambulatory ability (e.g., Expanded
Disability Status Scale score ≤5)
Yes: Go to #5
No: Pass to RPh.
Deny; medical
appropriateness
5. Does the patient have hepatic disease or
hepatic impairment, including ALT or AST
≥2-times the upper limit of normal, or
have a history of auto-immune hepatitis?
Yes: Pass to RPh. Deny;
medical appropriateness
No: Go to #6
6. Is the prescriber a neurologist who
regularly treats RMS?
Yes: Approve 150 mg once
monthly for 6 months
No: Pass to RPh.
Deny; medical
appropriateness
P&T/DUR Review: 1/17 (DM)
Implementation: 4-1-17

Oregon Medicaid PA Criteria 63 July 1, 2017
Dalfampridine
Goal(s):
To ensure appropriate drug use and limit to patient populations in which the drug has been
shown to be effective and safe.
Length of Authorization:
Up to 12 months
Requires PA:
Dalfampridine
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Does the patient have a diagnosis of
Multiple Sclerosis?
Yes: Go to #3
No: Pass to RPh. Deny;
medical appropriateness
3. Is the medication being prescribed by or in
consultation with a neurologist?
Yes: Go to #4
No: Pass to RPh. Deny;
medical appropriateness
4. Is the request for continuation of therapy
previously approved by the FFS program
(patient has completed 2-month trial)?
Yes: Go to Renewal
Criteria
No: Go to #5
5. Does the patient have a history of
seizures?
Yes: Pass to RPh. Deny;
medical appropriateness
No: Go to #6
6. Does the patient have moderate or severe
renal impairment (est. GFR <50 mL/min)?
Yes: Pass to RPh. Deny;
medical appropriateness
No: Go to #7
7. Is the patient ambulatory with a walking
disability requiring use of a walking aid
OR;
have moderate ambulatory dysfunction
and does not require a walking aid AND
able to complete the baseline timed 25-
foot walk test between 8 and 45 seconds?
Yes: Approve initial fill for
2-month trial.
No: Pass to RPh. Deny;
medical appropriateness

Oregon Medicaid PA Criteria 64 July 1, 2017
Renewal Criteria
1. Has the patient been taking dalfampridine
for ≥2 months with documented
improvement in walking speed while on
dalfampridine ( ≥20% improvement in
timed 25-foot walk test)?
Yes: Go to #2
No: Pass to RPh. Deny;
medical appropriateness
2. Is the medication being prescribed by or in
consultation with a neurologist?
Yes: Approve for 12
months
No: Pass to RPh. Deny;
medical appropriateness
Clinical Notes:
Because fewer than 50% of MS patients respond to therapy and therapy has risks, a trial of therapy should be
used prior to beginning ongoing therapy.
The patient should be evaluated prior to therapy and then 4 weeks to determine whether objective improvements
which justify continued therapy are present (i.e. at least a 20% improvement from baseline in timed walking
speed).
Dalfampridine is contraindicated in patients with moderate to severe renal impairment.
Dalfampridine can increase the risk of seizures; caution should be exercised when using concomitant drug
therapies known to lower the seizure threshold.
P&T Review: 5/16 (DM); 3/12
Implementation: 8/16, 9/1/13

Oregon Medicaid PA Criteria 65 July 1, 2017
Dispense as Written-1 (DAW-1) Reimbursement Rate
Brand Name and Multi-Source
Goal(s):
State compliance with US CFR 42 Ch.IV §447.512
Encourage use of generics.
Cover multi-source brand drugs at the higher reimbursement rate (DAW-1) only when
diagnosis is covered by OHP and medically necessary.
Length of Authorization:
Up to 12 months
Requires PA:
All brand multi-source drugs dispensed with a DAW-1 code (except narrow therapeutic index
drugs listed below) as defined in ORS 414.325.
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org
Prior Authorization is NOT required when multi-source brands are dispensed with DAW codes
other than DAW-1 and thus pay at generic AAAC (Average Actual Acquisition Cost).
AAAC prices and dispute forms are listed at:
http://www.oregon.gov/oha/pharmacy/Pages/aaac-rates.aspx
Narrow-therapeutic Index Drugs that
WILL PAY Without Prior Authorization
HSN
Generic Name
Brand Name
001893
Carbamazepine
Tegretol
004834
Clozapine
Clozaril
004524
Cyclosporine
Sandimmune
010086
Cyclosporine, modified
Neoral
000004
Digoxin
Lanoxin
002849
Levothyroxine
Levothroid, Synthroid
008060
Pancrelipase
Pancrease
001879
Phenytoin
Dilantin
002812
Warfarin
Coumadin
008974
Tacrolimus
Prograf
000025
Theophylline controlled-release
Various
HIC3-C4G
Insulin(s)
Various
Oregon Medicaid PA Criteria 66 July 1, 2017
Approval Criteria
1. Is the diagnosis an OHP (DMAP) above the
line diagnosis?
Yes: Go to #2.
No: Pass to RPH; Deny
(Not Covered by the
OHP). Offer alternative
of using generic or
pharmacy accepting
generic price (no DAW-
1)
2. Is the drug requested an antiepileptic in Std
TC 48 (e.g. Lamotrigine) or
immunosuppressant in Spec TC Z2E (e.g.
Cellcept) and is the client stabilized on the
branded product?
Yes: Document prior
use and approve for one
year.
No: Go to #3.
3. Does client have documented failure (either
therapeutic or contraindications) on an AB-
rated generic? (usually 2 weeks is
acceptable)
Yes: Document date
used and results of trial.
Approve for one year.
No: Pass to RPH;
Deny,
(Cost Effectiveness)
P&T / DUR Action: 2/23/06, 3/19/09, 12/3/09 (KK)
Implementation: 10/15, 7/1/06, 9/08, 7/1/09 (KK), 1/1/10 (KK)

Oregon Medicaid PA Criteria 67 July 1, 2017
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
Goal(s):
Promote cost-effective and safe step-therapy for management of type 2 diabetes mellitus (T2DM).
Length of Authorization:
Up to 12 months
Requires PA:
All DPP-4 inhibitors
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Does the patient have a diagnosis of Type 2
diabetes mellitus?
Yes: Go to #3
No: Pass to RPh. Deny;
medical
appropriateness
3. Has the patient tried and failed metformin
and a sulfonylurea, or have
contraindications to these treatments?
(document contraindication, if any)
Yes: Go to #4
No: Pass to RPh; deny
and recommend trial of
metformin or
sulfonylurea. See below
for metformin titration
schedule.
4. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class
No: Approve for up to
12 months
Initiating Metformin
1. Begin with low-dose metformin (500 mg) taken once or twice per day with meals (breakfast and/or dinner) or 850 mg once per
day.
2. After 5-7 days, if gastrointestinal side effects have not occurred, advance dose to 850 mg, or two 500 mg tablets, twice per day
(medication to be taken before breakfast and/or dinner).
3. If gastrointestinal side effects appear with increasing doses, decrease to previous lower dose and try to advance the dose at a later
time.
4. The maximum effective dose can be up to 1,000 mg twice per day. Modestly greater effectiveness has been observed with doses
up to about 2,500 mg/day. Gastrointestinal side effects may limit the dose that can be used.
Nathan, et al. Medical management of hyperglycemia in Type 2 Diabetes: a consensus algorithm for the initiation and adjustment of
therapy. Diabetes Care. 2008; 31;1-11.
P&T/DUR Review: 9/16 (KS); 9/15; 9/14; 9/13; 4/12; 3/11
Implementation: 10/13/16; 10/15; 1/15; 9/14; 1/14; 2/13

Oregon Medicaid PA Criteria 68 July 1, 2017
Dronabinol (Marinol®)
Goal(s):
Cover drugs only when used for covered OHP diagnoses, and restrict use to instances where
medical evidence supports use (e.g. Nausea associated with chemotherapy).There is limited
medical evidence supporting the use of dronabinol for many conditions.
Length of Authorization:
6 months to lifetime (criteria specific)
Requires PA:
Dronabinol (Marinol®)
Quantity Limits:
2.5mg & 5 mg = 3 units per day
10mg = 2 units per day
Apply ONLY to HIV/AIDS related anorexia and Non-Oncology related antiemetic use. No quantity
limits apply for Oncology (cancer) related antiemetic use.
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org
Metoclopramide (Reglan®)
Prochlorperazine (Compazine®)
Promethazine (Phenergan®)
5 HT3 antagonists (Zofran®, Anzemet®, or Kytril®) – authorized for >3 days
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Does client have diagnosis of anorexia
associated with AIDS? HIV?
Yes: Approve for lifetime
(until 12-31-2036). Apply
quantity limit (Anorexia
associated with
AIDS/HIV)
No: Go to #3.
3. Does client have current diagnosis of cancer
AND receiving chemotherapy or radiation
therapy?
Yes: Approve for length
of chemo or radiation
therapy.
No quantity limit.
(Chemotherapy or
Radiation, whichever is
applicable)
No: Go to #4.
4. Does client have refractory nausea that
would require hospitalization or ER visits?
Yes: Go to #5.
No: Go to #7.

Oregon Medicaid PA Criteria 69 July 1, 2017
Approval Criteria
5. Has client tried two medications listed
below?
Generic Name
Brand Name
Metoclopramide
Reglan®
Prochlorperazine
Compazine®
Promethazine
Phenergan®
5 HT3 drugs - Zofran®, Anzemet®, Kytril®
Yes: Approve for up to
six months. Apply
quantity limit (Refractory
Nausea With Failure of
Alternative Meds)
No: Go to #6.
6. Does client have contraindications, such as
allergies, or other reasons they CANNOT
use these anti-emetics? Document reason.
Yes: Approve for up to
six months. Apply
quantity limit (Refractory
Nausea With
Contraindication of
Alternative Meds)
No: Go to #7.
7. Does client have ONE of more of following
diagnosis? Cancer associated anorexia,
dystonic disorders, glaucoma, migraine,
multiple sclerosis, pain.
Yes: Pass to RPH;
Deny, (Medical
Appropriateness)
No: Pass to RPH; Go to
#8.
8. RPH only
All other indications need to be evaluated to
see if they are above or below the line
Above: Deny, (Medical
Appropriateness)
Below: Deny, (Not-
Covered by the OHP)
P&T / DUR Action: 2/23/06, 2/24/04, 2/11/03
Implementation: 10/15, 7/1/06, 5/31/05

Oregon Medicaid PA Criteria 70 July 1, 2017
Droxidopa (Northera®)
Goal(s):
To optimize appropriate pharmacological management of symptomatic neurogenic orthostatic
hypotension.
Length of Authorization:
Initial: 14 days
Renewal: 3 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the treated diagnosis on OHP funded
condition?
Yes: Go to #3.
No: Pass to RPH. Deny
for medical
appropriateness.
3. Does the patient have a diagnosis of
symptomatic orthostatic hypotension (ICD10
I951) due to primary autonomic failure
(Parkinson’s disease, multiple system
atrophy or pure autonomic failure),
dopamine beta-hydroxylase deficiency, or
nondiabetic autonomic neuropathy? (ICD10
G20; G230-232, G238; E700,E7021-7030,
E705,E708,E710, E7040,E71120,E7119,
E712, E7210, E7211,E7219, E7200-7201,
E7204, E7209, E7220, E7222, E7223,
E7229, E723, E728; G9001,G904, G909,
G9009, G9059, G90519, G90529, G990)
Yes: Go to #4.
No: Pass to RPH. Deny
for medical
appropriateness.
4. Is the patient currently receiving
antihypertensive medication?
Yes: Pass to RPH. Deny
for medical
appropriateness.
No: Go to #5.

Oregon Medicaid PA Criteria 71 July 1, 2017
Approval Criteria
5. Does the patient have a documented trial of
appropriate therapy with both
fludrocortisone and midodrine?
Message:
Preferred products are evidence-based
reviewed for comparative effectiveness and
safety by the Pharmacy and Therapeutics
Committee.
Yes: Approve for up to
14 days.
No: Inform provider
fludrocortisone and
midodrine are both
covered alternatives. If
justification provided for
not trying alternatives
(contraindications,
concern for adverse
effects, etc.), approve
for up to 14 days.
Renewal Criteria
1. Is this the first time the patient is requesting
this renewal?
Yes: Go to #2.
No: Approve for
up to 3 months.
2. Does the patient have documented
response to therapy (e.g., improvement in
dizziness/ lightheadedness)?
Yes: Approve for
up to 3 months.
No: Pass to
RPH; Deny for medical
appropriateness.
P&T / DUR Action: 1/29/15 (AG)
Implementation: 10/15

Oregon Medicaid PA Criteria 72 July 1, 2017
Drugs for Constipation
Length of Authorization:
Up to 6 months
Not Covered by OHP:
Disorders of function of stomach and other functional digestive disorders which includes
constipation and Irritable Bowel Syndrome (ICD-10: K3183-3184, K310, R1110, K30, K3189,
K319, K314-315, K312, K589, K591, K594, K5900-5902, K5909, K910-911, K9189, K598-599,
R159, R150, R152)
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the diagnosis covered by the OHP?
Yes: Go to 3
No: Pass to RPh. Deny;
diagnosis not covered
by OHP.
3. Will the prescriber consider a change to a
preferred product?
Message: preferred products do not require a
PA.
Yes: Inform prescriber
of covered alternatives
No: Go to 4
4. Has the patient failed a 2-week trial of at
least 3 of the following management
strategies due to lack of effectiveness,
contraindications or adverse effects?
A
Dietary modification—increased dietary
fiber (25 g/day)
B
Bulk-forming Laxatives: (psyllium [e.g,.
Metamucil],methylcellulose [e.g., Citrucel],
calcium carbophil [e.g., Fibercon])
C
Saline Laxatives: (magnesium hydroxide
[e.g., Milk of Magnesia], magnesium citrate,
sodium phosphate [Fleet Enema])
D
Stimulant Laxatives: (senna or bisacodyl)
E
Osmotic Laxatives: (lactulose, sorbitol or
polyethylene glycol 3350 [e.g., Miralax,
Glycolax])
Yes: Approve for 6
months.
No: Pass to RPh. Go to
5.

Oregon Medicaid PA Criteria 73 July 1, 2017
Approval Criteria
5. RPh only:
Constipation is not covered under the OHP. Therefore, funding for drugs that treat constipation
are dependent whether the constipation adversely affects, or is secondary to, the underlying
medical condition covered by the Prioritized List.
Alvimopan (ENTEREG): FDA labeling, including a black boxed warning for risk of
myocardial infarction, limit use to in hospital use only for a maximum of 15 doses. Evidence
is primarily for the immediate post-operative period only.
Linaclotide (LINZESS): Constipation secondary to irritable bowel syndrome is not
approvable. Chronic constipation caused by a funded condition or adversely affecting a
funded condition is approvable if medically appropriate and justification is provided for not
meeting criterion #4.
Lubiprostone (AMITIZA): Constipation secondary to irritable bowel syndrome or opioid-
induced constipation is not approvable. Chronic constipation caused by a funded condition
or adversely affecting a funded condition is approvable if medically appropriate and
justification is provided for not meeting criterion #4.
Methylnaltrexone (RELISTOR): Opioid-induced constipation in patients with non-cancer
pain is not approvable. Chronic constipation secondary to continuous opioid use as part of
a palliative care regimen is approvable if justification is provided for not meeting criterion
#4.
Naloxegol (MOVANTIK): Opioid-induced constipation in patients with non-cancer pain is
not approvable. Justification must be provided for not meeting criterion #4.
P&T Review: 3/15 (AG); 3/09
Implementation: 5/1/16; 10/15, 4/18/15

Oregon Medicaid PA Criteria 74 July 1, 2017
Drugs Selected for Manual Review by Oregon Health Plan
Goal:
Require specialty drugs selected by the Oregon Pharmacy & Therapeutics (P&T) Committee to be
manually reviewed and approved by the Oregon Health Plan (OHP) Medical Director.
Length of Authorization:
To be determined by OHP Medical Director.
Requires PA:
A drug approved by the P&T Committee to be manually reviewed by the OHP Medical Director for
approval.
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Pass to RPh. Deny; requires manual review and approval by the OHP Medical Director.
Message: The P&T Committee has determined this drug requires manual review by the OHP
Medical Director for approval.
P&T / DUR Review: 11/15 (AG)
Implementation 1/1/16

Oregon Medicaid PA Criteria 75 July 1, 2017
Drugs for Non-funded Conditions
Goal:
Restrict use of drugs reviewed by the Oregon Pharmacy & Therapeutics (P&T) Committee without
evidence for use in Oregon Health Plan (OHP)-funded conditions.
Length of Authorization:
Up to 6 months.
Requires PA:
A drug restricted by the P&T Committee due to lack of evidence for conditions funded by the OHP.
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the drug being used to treat an OHP-
funded condition?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP.
3. Pass to RPh. The prescriber must provide documentation of therapeutic failure, adverse event, or
contraindication alternative drugs approved by FDA for the funded condition. Otherwise, the
prescriber must provide medical literature supporting use for the funded condition. RPh may use
clinical judgement to approve drug for up to 6 months or deny request based on documentation
provided by prescriber.
P&T / DUR Review: 11/15 (AG)
Implementation 1/1/16

Oregon Medicaid PA Criteria 76 July 1, 2017
Erythropoiesis Stimulating Agents (ESAs)
Goal(s):
Cover ESAs according to OHP guidelines and current medical literature.
Cover preferred products when feasible.
Length of Authorization:
12 weeks initially, then up to 12 months
Quantity limit of 30 day per dispense
Requires PA:
All ESAs require PA for clinical appropriateness.
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is this an OHP covered diagnosis?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP
3. Is this continuation of therapy previously
approved by the FFS program?
Yes: Go to #12
No: Go to #4
4. Is the requested product preferred?
Yes: Go to #6
No: Go to #5
5. Will the prescriber change to a preferred
product?
Message:
Preferred products do not require PA.
Preferred products are evidence-based
reviewed for comparative effectiveness
and safety by the Pharmacy and
Therapeutics (P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Go to #6
6. Is the diagnosis anemia due to chronic renal
failure
1
or chemotherapy
2,3
?
Yes: Go to #7
No: Go to #8
7. Is Hgb <10 g/dL or Hct <30%
AND
Transferrin saturation >20% and/or ferritin
>100 ng/mL?
Yes: Approve for 12
weeks with additional
approval based upon
adequate response.
No: Pass to RPh. Deny;
medical
appropriateness
8. Is the diagnosis anemia due to HIV
4
?
Yes: Go to #9
No: Go to #10

Oregon Medicaid PA Criteria 77 July 1, 2017
Approval Criteria
9. Is the Hgb <10 g/dL or Hct <30%
AND
Transferrin saturation >20%
AND
Endogenous erythropoietin <500 IU/L
AND
If on zidovudine, is dose <4200 mg/week?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness
10. Is the diagnosis anemia due to ribavirin
treatment
5
?
Yes: Go to #11
No: Pass to RPh. Deny;
medical
appropriateness
11. Is the Hgb <10 g/dL or Hct <30%
AND
Is the transferrin saturation >20% and/or
ferritin >100 ng/mL
AND
Has the dose of ribavirin been reduced by
200 mg/day and anemia persisted >2
weeks?
Yes: Approve up to the
length of ribavirin
treatment.
No: Pass to RPh. Deny;
medical
appropriateness
12. Has the patient responded to initial therapy?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness
References:
1. National Kidney Foundation. NKF KDOQI Guidelines. NKF KDOQI Guidelines 2006. Available at:
http://www.kidney.org/professionals/KDOQI/guidelines_anemia/index.htm . Accessed May 25, 2012.
2. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of
Hermatology Clinical Practice Guideline Update on the Use of Epoetin and Darbepoetin in Adult
Patients With Cancer. JCO 2010:28(33):4996-5010. Available at: www.asco.org/institute-quality/asco-
ash-clinical-practice-guideline-update-use-epoetin-and-darbepoetin-adult. Accessed May 1, 2012.
3. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical
Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with
cancer. Blood. 2010:116(20):4045-4059.
4. Volberding PA, Levine AM, Dieterich D, et al. Anemia in HIV infection: Clinical Impact and Evidence-
Based Management Strategies. Clin Infect Dis. 2004:38(10):1454-1463. Available at:
http://cid.oxfordjournals.org/content/38/10/1454. Accessed May 8, 2012.
5. Recombinant Erythropoietin Criteria for Use for Hepatitis C Treatment-Related Anemia. VHA Pharmacy
Benefits Management Strategic Healthcare Group and Medical Advisory Panel. April 2007
P&T Review: 7/16 (DM); 5/14; 11/12; 6/12; 2/12, 9/10
Implementation: 10/13/16; 1/1/13; 9/24/12; 5/14/12

Oregon Medicaid PA Criteria 78 July 1, 2017
Estrogen Derivatives
Goal(s):
Restrict use to medically appropriate conditions funded under the OHP
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred estrogen derivatives
All estrogen derivatives for patients <18 years of age
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the estrogen requested for a patient ≥18
years old?
Yes: Go to #3
No: Go to #4
3. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require a co-
pay. Preferred products are evidence-
based reviewed for comparative
effectiveness and safety by the Oregon
Pharmacy & Therapeutics (P&T)
Committee.
Yes: Inform prescriber
of covered alternatives
in class and approve for
up to 12 months.
No: Approve for up to
12 months.
4. Is the medication requested for gender
dysphoria (ICD10 F642, F641)?
Yes: Go to #5
No: Go to #6
5. Have all of the following criteria been met?
Patient has the capacity to make fully
informed decisions and to give consent
for treatment; and
If patient <18 years of age, the prescriber
is a pediatric endocrinologist; and
The prescriber agrees criteria in
Guideline Notes on the OHP List of
Prioritized Services have been met.
Yes: Approve for up to 6
months
No: Pass to RPh. Deny;
medical
appropriateness
6. Is the medication requested for
hypogonadism?
Yes: Approve for up to 6
months
No: Go to #7

Oregon Medicaid PA Criteria 79 July 1, 2017
Approval Criteria
7. RPh only: All other indications need to be
evaluated to see if funded under the OHP.
If funded and prescriber
provides supporting
literature: Approve for up
to 12 months.
If non-funded: Deny; not
funded by the OHP
P&T / DUR Review: 1/17 (SS); 11/15 (KS)
Implementation: 4/1/17; 1/1/16

Oregon Medicaid PA Criteria 80 July 1, 2017
Exclusion List
Deny payment for drug claims for drugs that are only FDA-approved for indications that are not
covered by the Oregon Health Plan (OHP).
Other exclusionary criteria are in rules at:
www.oregon.gov/OHA/healthplan/pages/pharmacy-policy.aspx
Excerpt from
OAR 410-121-0147 Exclusions and Limitations
(DMAP Pharmaceutical Services Program)
1) The following items are not covered for payment by the Division of Medical Assistance Programs
(DMAP) Pharmaceutical Services Program:
(a) Drug products for diagnoses below the funded line on the Health Services Commission Prioritized
List or an excluded service under Oregon Health Plan (OHP) coverage;
(b) Home pregnancy kits;
(c) Fluoride for individuals over 18 years of age;
(d) Expired drug products;
(e) Drug products from non-rebatable manufacturers, with the exception of selected oral nutritionals,
vitamins, and vaccines;
(f) Active Pharmaceutical Ingredients (APIs) and Excipients as described by Centers for Medicare
and Medicaid (CMS);
(g) Drug products that are not assigned a National Drug Code (NDC) number;
(h) Drug products that are not approved by the Food and Drug Administration (FDA);
(i) Drug products dispensed for Citizen/Alien-Waived Emergency Medical client benefit type;
(j) Drug Efficacy Study Implementation (DESI) drugs (see OAR 410-121-0420);
(k) Medicare Part D covered drugs or classes of drugs for fully dual eligible clients (see OAR 410-
121-0149, 410-120-1200, & 410-120-1210).
NOTE: Returns as “70 – NDC NOT COVERED”
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. For what reason is it being rejected?
3. “70” NDC Not Covered (Transaction line
states “Bill Medicare”
Yes: Go to the Medicare
B initiative in these
criteria.
No: Go to #2B
4. “70” NDC Not Covered (Transaction line
states “Bill Medicare or Bill Medicare D”
Yes: Informational Pa to
bill specific agency
No: Go to #2C

Oregon Medicaid PA Criteria 81 July 1, 2017
Approval Criteria
5. “70” NDC Not Covered (due to expired or
invalid NDC number)
Yes: Informational PA
with message “The drug
requested does not have
a valid National Drug
Code number and is not
covered by Medicaid.
Please bill with correct
NDC number.”
No: Go to #2D
6. “70” NDC Not Covered (due to DME items,
excluding diabetic supplies) (Error code M5
–requires manual claim)
Yes: Informational PA
(Need to billed via DME
billing rules)
1-800-336-6016
No: Go to #2E
7. “70” NDC Not Covered (Transaction line
states “Non-Rebatable Drugs” )
Yes: Pass to RPh. Deny
(Non-Rebatable Drug)
with message
“The drug requested is
made by company that
does not participate in
Medicaid Drug Rebate
Program and is
therefore not covered”
No: Go to #2F
8. “70” NDC Not Covered (Transaction line
states “DESI Drug”)
Yes: Pass to RPh. Deny
(DESI Drug) with
message,
“The drug requested is
listed as a “Less-Than-
Effective Drug” by the
FDA and not covered by
Medicaid.”
No: Pass to RPh. Go to
#3

Oregon Medicaid PA Criteria 82 July 1, 2017
Approval Criteria
9. RPh only: “70” NDC Not Covered (Drugs on
the Exclusion List) All indications need to be
evaluated to see if they are above the line or
below the line.
Above: Deny with
yesterday’s date
(Medically
Appropriateness) and
use clinical judgment to
APPROVE for 1 month
starting today to allow
time for appeal.
Message: “Although the
request has been denied
for long term use
because it is considered
medically inappropriate,
it has also been
APPROVED for one
month to allow time for
appeal.”
Below: Deny. Not
funded by the OHP.
Message: “The
treatment for your
condition is not a
covered service on the
Oregon Health Plan.”
If the MAP desk notes a drug is often requested for a covered indication, notify Lead Pharmacist so that policy changes
can be considered for valid covered diagnoses.
Exclusion List
Drug Code
Description
DMAP Policy
DCC = 1
Drugs To Treat Impotency/
Erectile Dysfunction
Impotency Not Covered on OHP
List
DCC = B
Fertility Agents
Fertility Treatment Not Covered
on OHP List
DCC = D
Diagnostics
DME Billing Required
DCC= F, except HSN =
018751
002111
002112
002070
002113
016924
Weight Loss Drugs
Weight Loss Not Covered on
OHP List except In cases of co-
morbidity. Exceptions are Prior
Authorized
DCC= Y
Ostomy Supplies
DME Billing Required
HIC3= B0P
Inert Gases
DME Billing Required
HIC3= L1C
Hypertrichotic Agents,
Systemic/Including
Combinations
Cosmetic Indications Not
Covered on OHP List
HIC3= Q6F
Contact Lens Preparations
Cosmetic Indications Not
Covered on OHP List
HIC3=X1C
IUDs
DME Billing Required
HIC3=D6C
Alosetron Hcl
IBS Not Covered on OHP List
HIC3=D6E
Tegaserod
IBS Not Covered on OHP List
HIC3=L1D
Hyperpigmentation Agents
Drug Code
Description
DMAP Policy

Oregon Medicaid PA Criteria 83 July 1, 2017
HIC3=L3P
Astringents
HIC3=L4A
Topical Antipruritic Agents
HIC3=L5A;
Except HSN=
002466
006081 (Podophyllin Resin)
Keratolytics
Acne, Warts, Corns/Calluses;
Seborrhea Are Not Covered on
OHP List
HIC3=L5B
Sunscreens
Cosmetic Indications, Acne,
Atopic Dermatitis, Warts,
Corns/Callouses; Diaper Rash,
Seborrhea Are Not Covered on
OHP List
HIC3=L5C
Abrasives
Cosmetic Indications, Acne,
Atopic Dermatitis, Warts,
Corns/Callouses; Diaper Rash,
Seborrhea Are Not Covered on
OHP List
HIC3=L5E
Anti Seborrheic Agents
Seborrhea Not Covered on OHP
List
HIC3=L5G
Acne Agents
Acne Not Covered on OHP List
HIC3=L5H
Acne Agents, Topical
Acne Not Covered on OHP List
HIC3=L6A;
Except HSN = 002577
002576
002574
002572 (Capsaicin)
Irritants
Acne, Atopic Dermatitis,
Seborrhea, Sprains Not
Covered on OHP List
HIC3=L7A
Shampoos
Cosmetic Indications,
Seborrhea, Not Covered on
OHP List
HIC3=L8A
Deodorants
Cosmetic Indications Not
Covered on OHP List
HIC3=L8B
Antiperspirants
Cosmetic Indications Not
Covered on OHP List
HIC3=L9A
Topical Agents, Misc
Cosmetic Indications, Acne,
Atopic Dermatitis, Warts,
Corns/Callouses; Diaper Rash,
Seborrhea, are Not Covered on
OHP List
HIC3=L9B
Vit A Used for Skin
Acne Not Covered on OHP List
HIC3=L9C
Antimelanin Agents
Pigmentation Disorders Not
Covered on OHP List
HIC3=L9D
Topical Hyperpigmentation
Agent
Pigmentation Disorders Not
Covered on OHP List
HIC3=L9F
Topical Skin Coloring Dye Agent
Cosmetic Indications Not
Covered on OHP List
HIC3=L9I
Topical Cosmetic Agent; Vit A
Cosmetic Indications Not
Covered on OHP List
HIC3=L9J
Hair Growth Reduction Agents
Cosmetic Indications Not
Covered on OHP List
Drug Code
Description
DMAP Policy
HIC3=Q5C
Topical Hypertrichotic Agents
Cosmetic Indications Not

Oregon Medicaid PA Criteria 84 July 1, 2017
Covered on OHP List
HIC3=Q5K
Topical Immunosuppressants
Atopic Dermatitis Not Covered
on OHP List
HIC3=Q6R, Q6U, Q6D
Antihistamine-Decongestant,
Vasoconstrictor and Mast Cell
Eye Drops
Allergic Conjunctivitis Not
Covered on OHP List
HIC3= U5A, U5B, U5F & S2H
plus HSN= 014173
Herbal Supplements “ Natural
Anti-Inflammatory Supplements”
- Not Including Nutritional
Supplements such as: Ensure,
Boost, Etc.
HSN = 004045 +
ROA = TOPICAL
Clindamycin Topical
Acne Not Covered on OHP List
HSN=003344
Sulfacetamide Sodium/Sulfur
Topical
Acne Not Covered on OHP List
HSN=008712, 004022 +
ROA=TOPICAL
Erythromycin Topical
Acne Not Covered on OHP List
HSN=025510
Rosacea
Acne Not Covered on OHP List
TC=93;
Except HSN =
002363 (dextranomer)
002361 (zno)
Emollients/Protectants
Cosmetic Indications, Acne,
Atopic Dermatitis, Warts,
Corns/Callouses; Diaper Rash,
Seborrhea, Psoriasis Are Not
Covered on OHP List
P&T Review: 2/23/06
Implementation: 5/1/16; 9/1/06; 1/1/12

Oregon Medicaid PA Criteria 85 July 1, 2017
Fidaxomicin (Dificid®)
Goal(s):
To optimize appropriate treatment of Clostridium difficile-associated infection.
Length of Authorization:
10 days
Requires PA:
Fixaxomicin
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Does the patient have a diagnosis of
Clostridium difficile-associated infection
(CDI)? (ICD-10 A047
Yes: Go to #3.
No: Pass to RPH;
Deny (medical
appropriateness)
3. Will the prescriber consider changing to a
preferred antibiotic?
Message:
• Preferred products are evidence-based
reviewed for comparative effectiveness
and safety by the Pharmacy and
Therapeutics Committee.
Yes: Inform Provider of
covered alternatives in
class.
No: Go to #4
4. Does the patient have a documented trial of
appropriate therapy with vancomycin or
metronidazole for a first recurrence or
contraindication to therapy?
Yes: Go to #5.
No: Pass to RPH;
Deny (medical
appropriateness)
5. Does the patient have severe, complicated
CDI (life-threatening or fulminant infection or
toxic megacolon)?
Yes: Pass to RPH;
Deny (medical
appropriateness)
No: Approve for up to
10 days
P&T / DUR Review: 5/15 (AG); 4/12
Implementation: 10/15; 7/12

Oregon Medicaid PA Criteria 86 July 1, 2017
Glucagon-like Peptide-1 (GLP-1) Receptor Agonists
Goal(s):
Promote cost-effective and safe step-therapy for management of type 2 diabetes mellitus (T2DM).
Length of Authorization:
Up to 12 months
Requires PA:
All GLP-1 receptor agonists
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Does the patient have a diagnosis of Type 2
diabetes mellitus?
Yes: Go to #3
No: Pass to RPh.
Deny; medical
appropriateness.
3. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require PA.
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class
No: Go to #4
4. Has the patient tried and failed metformin and
sulfonylurea therapy or have contraindications
to these treatments?
(document contraindication, if any)
Yes: Yes: Approve for
up to 12 months
No: Pass to RPh.
Deny; medical
appropriateness.
Recommend trial of
metformin or
sulfonylurea. See
below for metformin
titration schedule.
Initiating Metformin
1. Begin with low-dose metformin (500 mg) taken once or twice per day with meals (breakfast and/or dinner) or 850 mg
once per day.
2. After 5-7 days, if gastrointestinal side effects have not occurred, advance dose to 850 mg, or two 500 mg tablets,
twice per day (medication to be taken before breakfast and/or dinner).
3. If gastrointestinal side effects appear with increasing doses, decrease to previous lower dose and try to advance the
dose at a later time.
4. The maximum effective dose can be up to 1,000 mg twice per day. Modestly greater effectiveness has been observed

Oregon Medicaid PA Criteria 87 July 1, 2017
with doses up to about 2,500 mg/day. Gastrointestinal side effects may limit the dose that can be used.
Nathan, et al. Medical management of hyperglycemia in Type 2 Diabetes: a consensus algorithm for the initiation and
adjustment of therapy. Diabetes Care. 2008; 31;1-11.
P&T Review: 1/17 (KS); 11/16; 9/16; 9/15; 1/15; 9/14; 9/13; 4/12; 3/11
Implementation: 4/1/17; 2/15; 1/14

Oregon Medicaid PA Criteria 88 July 1, 2017
Gonadotropin-Releasing Hormone (GnRH) Analogs
Goal(s):
Restrict pediatric use to medically appropriate conditions funded under the Oregon Health Plan
(eg, central precocious puberty or gender dysphoria)
Length of Authorization:
Up to 6 months
Requires PA:
GnRH analogs (i.e., goserelin, histrelin, leuprolide, nafarelin, triptorelin) prescribed for pediatric
patients less than 18 years of age.
Approval Criteria
1. What diagnosis is being treated and what is
the age and gender of the patient assigned
at birth?
Record ICD10 code.
Record age and gender assigned at birth
2. Is the prescriber a pediatric endocrinologist?
Yes: Go to #3
No: Pass to RPh; deny
for medical
appropriateness
3. Is the diagnosis central precocious puberty
(ICD10 E301, E308) or other endocrine
disorder (E34.9)?
Yes: Approve for up to 6
months
No: Go to #4
4. Is the diagnosis gender dysphoria (ICD10
F642, F641)?
Yes: Go to #5
No: Pass to RPh; go to
#6
5. Does the request meet all of the following
criteria?
Diagnosis of gender dysphoria made by
a mental health professional with
experience in gender dysphoria.
Onset of puberty confirmed by physical
changes and hormone levels, but no
earlier than Tanner Stages 2.
The prescriber agrees criteria in the
Guideline Notes on the OHP List of
Prioritized Services have been met.
Yes: Approve for up to 6
months
No: Pass to RPh; deny
for medical
appropriateness
6. RPh only:
All other indications need to be evaluated as to whether it is funded under the OHP. Refer unique
situations to Medical Director of DMAP.
P&T / DUR Review: 11/15 (KS); 7/15; 5/15; 9/07
Implementation: 1/1/16; 7/1/15; 11/07; 7/09

Oregon Medicaid PA Criteria 89 July 1, 2017
Agents for Gout
Goal(s):
To provide evidenced-based step-therapy for the treatment of acute gout flares, prophylaxis of
gout and chronic gout.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Will the provider switch to a preferred
product?
Note: Preferred products are reviewed for
comparative effectiveness and safety by the
Oregon Pharmacy and Therapeutics
Committee. Preferred products are available
without a PA
Yes: Inform prescriber
of covered alternatives
in the class
No: Go to #3
3. Is the request for colchicine?
Yes: Go to #4
No: Go to #5
4. Has the patient tried and failed NSAID
therapy or have contraindications to NSAIDs
or is a candidate for combination therapy
(i.e., multiple joint involvement and severe
pain)?
Yes: Approve for 12
months
No: Pass to RPh. Deny;
recommend trial of
NSAID
5. Is the request for febuxostat?
Yes: Go to #6
No: Go to #7
6. Has the patient tried and failed allopurinol or
has contraindications to allopurinol?
Yes: Approve for 12
months
NO: Pass to RPh.
Deny; recommend trial
of allopurinol
7. Is the request for lesinurad?
Yes: Go to #8
No: Pass to RPh. Deny;
Medical
appropriateness

Oregon Medicaid PA Criteria 90 July 1, 2017
Approval Criteria
8. Is the patient concomitantly taking a
xanthine oxidase inhibitor (e.g., allopurinol,
fubuxostat)?
Yes: Go to #9
No: Pass to RPh. Deny;
medical
appropriateness
9. Is the estimated CrCl < 45 mL/min?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Approve for 12
months at a maximum
daily dose of 200 mg
P&T/DUR Review: 1/17 (KS)
Implementation: 4/1/2017

Oregon Medicaid PA Criteria 91 July 1, 2017
Growth Hormones
Goal(s):
Restrict use of growth hormone (GH) for funded diagnoses where there is medical evidence of
effectiveness and safety.
NOTE: Treatment with growth hormone (GH) is included only for children with: pituitary dwarfism,
Turner’s syndrome, Prader-Willi-syndrome, Noonan’s syndrome, short stature homeobox-containing
gene (SHOX), chronic kidney disease (stage 3 or higher) and those with renal transplant. Treatment
with GH should continue only until adult height as determined by bone age is achieved. Treatment is
not included for isolated deficiency of human growth hormone or other conditions in adults.
Length of Authorization:
Up to 12 months
Requires PA:
All GH products require prior authorization for OHP coverage. GH treatment for adults is not
funded by the OHP.
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Initial Approval Criteria
1. What is the diagnosis being treated?
Record ICD10 code
2. Is the patient an adult (>18 years of age)?
Yes: Pass to RPh.
Deny; not funded by the
OHP
No: Go to #3
3. Is this a request for initiation of growth
hormone?
Yes: Go to #4
No: Go to Renewal
Criteria
4. Is the prescriber a pediatric endocrinologist
or pediatric nephrologist?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness
5. Is the diagnosis promotion of growth delay
in a child with 3rd degree burns?
Yes: Document and
send to DHS Medical
Director for review and
pending approval
No: Go to #6

Oregon Medicaid PA Criteria 92 July 1, 2017
Initial Approval Criteria
6. Is the diagnosis one of the following?
Turner’s syndrome (ICD10 Q969)
Noonan’s syndrome (ICD10 E7871-
7872, Q872-873, Q875, Q8781, Q8789,
Q898)
Prader-Willi syndrome (PWS) (ICD10
Q871)
Pituitary dwarfism (ICD10 E230)
Short stature homeobox-containing gene
(SHOX) (ICD10 R6252)
Chronic kidney disease (CKD, Stage ≥3)
(ICD10 N183-N185)
Renal transplant (ICD10 Z940)
Yes: Document and go
to #7
No: Pass to RPh. Deny;
not funded by the OHP.
7. If male, is bone age <16 years?
If female, is bone age <14 years?
Yes: Go to #8
No: Pass to RPh. Deny;
medical
appropriateness
8. Is there evidence of non-closure of
epiphyseal plate?
Yes: Go to #9
No: Pass to RPh. Deny;
medical
appropriateness
9. Is the product requested preferred?
Yes: Approve for up to
12 months
No: Go to #10
10. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class and approve for
up to 12 months.
No: Approve for up to
12 months
Renewal Criteria
1. Document approximate date of initiation of therapy and diagnosis (if not already done).
2. Is growth velocity greater than 2.5 cm per
year?
Yes: Go to #3
No: Pass to RPh.
Deny; medical
appropriateness
3. Is male bone age <16 years or female bone
age <14 years?
Yes: Go to #4
No: Pass to RPh.
Deny; medical
appropriateness
4. Is the product requested preferred?
Yes: Approve for up to
12 months
No: Go to #5

Oregon Medicaid PA Criteria 93 July 1, 2017
5. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber of
covered alternatives in
class and approve for up
to 12 months
No: Approve for up to
12 months
P&T Review: 9/16; 9/15; 9/14; 9/10; 5/10; 9/08; 2/06; 11/03; 9/03
Implementation: 10/13/16; 1/1/11, 7/1/10, 4/15/09, 10/1/03, 9/1/06; 10/1/03

Oregon Medicaid PA Criteria 94 July 1, 2017
Hepatitis B Antivirals
Goal(s):
Approve treatment supported by medical evidence and consensus guidelines
Cover preferred products when feasible for covered diagnosis
Length of Authorization:
Up to 12 months; quantity limited to a 30-day supply per dispensing.
Requires PA:
All Hepatitis B antivirals
Covered Alternatives:
Preferred alternatives listed at http://www.orpdl.org/drugs/
Pediatric Age Restrictions:
lamivudine (Epivir HBV) – 2-17 years
adefovir dipivoxil (Hepsera) – 12 years and up
entecavir (Baraclude) – 2 years and up
telbivudine (Tyzeka) –16 years and up
tenofovir disoproxil fumarate (Viread) – 12 years and up
tenofovir alafenamide (Vemlidy) – safety and effectiveness not established in pediatrics
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis an OHP-funded diagnosis?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP
3. Is the request for an antiviral for the
treatment of HIV/AIDS?
Yes: Approve for up to
12 months
No: Go to #4
4. Is the request for treatment of chronic
Hepatitis B?
Yes: Go to #5
No: Pass to RPh. Deny;
medical appropriateness

Oregon Medicaid PA Criteria 95 July 1, 2017
Approval Criteria
5. Is this a continuation of current therapy
previously approved by the FFS program (i.e.
filled prescription within prior 90 days)?
Verify via pharmacy claims.
***If request is for Pegasys, refer to PA
criteria “Pegylated Interferon and
Ribavirin.”***
Yes: Go to Renewal
Criteria
No: Go to #6
6. Has the client tried and is intolerant to,
resistant to, or has a contraindication to the
preferred products?
Yes: Document
intolerance or
contraindication.
Approve requested
treatment for 6 months
with monthly quantity
limit of 30-day supply.
No: Go to #7
7. Will the prescriber consider a change to a
preferred product?
Yes: Inform prescriber of
covered alternatives in
class
No: Approve requested
treatment for 6 months
with monthly quantity
limit of 30-day supply
Renewal Criteria
1. Is the patient adherent with the requested
treatment (see refill history)?
Yes: Go to #2
2. Is HBV DNA undetectable (below 10 IU/mL
by real time PCR) or the patient has
evidence of cirrhosis?
Note: Antiviral treatment is indicated
irrespective of HBV DNA level in patients
with cirrhosis to prevent reactivation.
Yes: Approve for up to 1 year with monthly quantity
limit of 30-day supply
P&T Review: 3/17(MH); 3/12
Implementation: 4/1/17; 5/29/14; 1/13

Oregon Medicaid PA Criteria 96 July 1, 2017
Hepatitis C Direct-Acting Antivirals
Goals:
Approve use of cost-effective treatments supported by the medical evidence.
Provide consistent patient evaluations across all hepatitis C treatments.
Ensure appropriate patient selection based on disease severity, genotype, and comorbidities.
Length of Authorization:
8-12 weeks
Requires PA:
All direct-acting antivirals for treatment of Hepatitis C
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the request for treatment of chronic
Hepatitis C infection?
Yes: Go to #3
No: Pass to RPh.
Deny; medical
appropriateness.
3. Is expected survival from non-HCV-
associated morbidities more than 1
year?
Yes: Go to #4
No: Pass to RPh.
Deny; medical
appropriateness.
4. Has all of the following pre-treatment
testing been documented:
a. Genotype testing in past 3 years;
b. Baseline HCV RNA level in past 6
months;
c. Current HIV status of patient
d. Current HBV status of patient
e. Pregnancy test in past 30 days for
a woman of child-bearing age;
and
f. History of previous HCV treatment
and outcome?
Note: Direct-acting antiviral agents can
re-activate hepatitis B in some patients.
Patients with history of HBV should be
monitored carefully during and after
treatment for flare-up of hepatitis.
Yes: Record results of each test
and go to #5
No: Pass to RPh.
Request updated
testing.

Oregon Medicaid PA Criteria 97 July 1, 2017
Approval Criteria
5. Has the patient failed treatment with
any of the following HCV NS5A
inhibitors:
a) Daclatasvir plus sofosbuvir;
b) Ledipasvir/sofosbuvir;
c) Paritaprevir/ritonavir/ombitasvir
plus dasabuvir;
d) Elbasvir/grazoprevir; or
e) Sofosbuvir/velpatasvir)?
Note: Patients who failed treatment
with sofosbuvir +/- ribavirin or
PEGylated interferon can be
retreated (see table below).
Yes: Pass to RPh. Deny;
medical appropriateness.
Note: If urgent retreatment is
needed, resistance testing must
be done to indicate susceptibility
to prescribed regimen.
Refer to medical director for
review.
No: Go to #6
6. Which regimen is requested?
Document and go to #7
7. Does the patient have HIV
coinfection AND: A biopsy, imaging
test (transient elastography
[FibroScan], acoustic radiation force
impulse imaging [ARFI], or shear
wave elastography [SWE], or serum
test if the above are not available
(enhanced liver fibrosis [ELF];
Fibrometer; FIBROSpect II) to
indicate fibrosis (METAVIR F2) AND
the patient is under treatment by a
specialist with experience in HIV?
Yes: Go to #12
Note: Other imaging and blood
tests are not recommended
based on evidence of poor
sensitivity and specificity
compared to liver biopsy.
Further information on coverage
guidance from the Health
Evidence Review Commission
(HERC) is available at
http://www.oregon.gov/oha/herc/
Pages/CompletedGuidances.asp
x
No: Go to #8

Oregon Medicaid PA Criteria 98 July 1, 2017
Approval Criteria
8. Does the patient have:
a) A biopsy, imaging test (transient
elastography [FibroScan
®
],
acoustic radiation force impulse
imaging [ARFI], or shear wave
elastography [SWE]) to indicate
advanced fibrosis (METAVIR F3)
or cirrhosis (METAVIR F4); or
Clinical, radiologic or laboratory
evidence of complications of
cirrhosis (ascites, portal
hypertension, hepatic
encephalopathy, hepatocellular
carcinoma)?
Yes: Go to #11
Note: Other imaging and blood
tests are not recommended
based on evidence of poor
sensitivity and specificity
compared to liver biopsy
Further information on coverage
guidance from the Health
Evidence Review Commission
(HERC) is available at
http://www.oregon.gov/oha/herc/
Pages/CompletedGuidances.asp
x
No: Go to #9
9. Does the patient have one of the
following extrahepatic manifestations
of Hepatitis C (with documentation
from a relevant specialist that their
condition is related to HCV)?
a) Type 2 or 3 cryoglobulinemia
with end-organ manifestations
(i.e., leukocytoclastic
vasculitis); or
b) Proteinuria, nephrotic
syndrome, or
membranoproliferative
glomerulonephritis; or
c) Porphyria cutanea tarda
Yes: Go to #11
No: Go to #10
10. Is the patient in one of the following
transplant settings:
a) Listed for a transplant and
treatment is essential to
prevent recurrent hepatitis C
infection post-transplant; or
b) Post solid organ transplant?
Yes: Go to #11
No: Pass to RPh.
Deny; medical
appropriateness.
Oregon Medicaid PA Criteria 99 July 1, 2017
Approval Criteria
11. If METAVIR F4: Is the regimen
prescribed by, or in consultation with,
a hepatologist, gastroenterologist, or
infectious disease specialist with
experience in treatment of Hepatitis
C? OR
If METAVIR F3: Is the regimen
prescribed by, OR is the patient in
the process of establishing care with,
a hepatologist, gastroenterologist, or
infectious disease specialist with
experience in the treatment of
Hepatitis C? OR
If METAVIR <F2: Is the regimen
prescribed by a provider
knowledgeable in the treatment of
Hepatitis C?
Yes: Go to #12
No: Pass to RPh.
Deny; medical
appropriateness.
Forward to DMAP for
further manual review
to determine
appropriateness of
prescriber.
12. In the previous 6 months:
Has the patient actively abused
alcohol (>14 drinks per week for
men or >7 drinks per week for
women or binge alcohol use (>4
drinks per occasion at least once
a month); OR
Has the patient been diagnosed
with a substance use disorder;
OR
Is the prescriber aware of current
alcohol abuse or illicit injectable
drug use?
Yes: Go to #13
No: Go to #14
13. Is the patient enrolled in a treatment
program under the care of an
addiction/substance use treatment
specialist?
Yes: Go to #14
No: Pass to RPh.
Deny; medical
appropriateness.
14. Do the patient and provider agree to
comply with all case management
interventions and adhere to
monitoring requirements required by
the Oregon Health Authority,
including measuring and reporting of
a post-treatment viral load?
Yes: Go to #15
No: Pass to RPh.
Deny; medical
appropriateness.

Oregon Medicaid PA Criteria 100 July 1, 2017
Approval Criteria
15. Is the prescribed drug:
a) Elbasvir/grazoprevir for GT 1a
infection; or
b) Daclatasvir + sofosbuvir for
GT 3 infection?
Yes: Go to #16
No: Go to #17
16. Has the patient had a baseline NS5a
resistance test show a resistant
variant to one of the agents in #16?
Yes: Pass to RPh; deny for
appropriateness
No: Go to #17
Baseline testing for
resistance variants is
required prior to
approval.
17. Is the prescribed drug regimen a
recommended regimen based on the
patient’s genotype and cirrhosis
status (Table 1)?
Yes: Approve for 8-24 weeks
based on duration of treatment
indicated for approved regimen
No: Pass to RPh.
Deny; medical
appropriateness.
Table 1: Recommended Treatment Regimens for Chronic Hepatitis C.
Genotype
Cirrhosis Status
Recommended Regimen^
Duration of Treatment
Genotype 1
Treatment-naïve
Non-cirrhotic
EBR/GZR
LDV/SOF
12 weeks except if LDV/SOF
and HCV RNA < 6 million IU/mL,
give for 8 weeks
Compensated
Cirrhosis
EBR/GZR
LDV/SOF
12 weeks
Decompensated
Cirrhosis
LDV/SOF + RBV
12 weeks
Treatment-
experienced*
Non-cirrhotic
EBR/GZR
LDV/SOF +/- RBV**
12 weeks
Compensated
Cirrhosis
EBR/GZR
LDV/SOF + RBV
12 weeks
12 weeks – 24 weeks
c
Decompensated
Cirrhosis
LDV/SOF + RBV
24 weeks
Genotype 2
Naïve or
Experienced
Non-cirrhotic
SOF/VEL
12 weeks
Compensated
Cirrhosis
SOF/VEL + RBV
**
12 weeks

Oregon Medicaid PA Criteria 101 July 1, 2017
Decompensated
Cirrhosis
SOF/VEL + RBV
12 weeks
Genotype 3
Naïve or
Experienced
Non-cirrhotic
LDV/SOF + RBV
SOF/VEL
12 weeks
Compensated
Cirrhosis
SOF/VEL + RBV
±
12 weeks
Decompensated
Cirrhosis
SOF/VEL + RBV
12 weeks
Genotype 4
Naïve or
Experienced
Non-cirrhotic
EBR/GZR
LDV/SOF
12 weeks
Compensated
Cirrhosis
EBR/GZR
LDV/SOF
12 weeks
Decompensated
Cirrhosis
LDV/SOF + RBV
12 weeks (24 weeks if prior
SOF treatment has failed)
Genotypes 5 and 6
Naïve or
Experienced
With or Without
Compensated
Cirrhosis
LDV/SOF
12 weeks
Abbreviations: EBV/GZR = elbasvir/grazoprevir (Zepatier
®
) ; LDV/SOF = ledipasvir and sofosbuvir (Harvoni
®
); RBV = ribavirin; SOF = sofosbuvir
(Sovaldi
®
); SOF/VEL = sofosbuvir/velptasvir (Epclusa
®
)
*Treatment-experienced defined as previous treatment with PEG/RBV or SOF/RBV only.
**RBV required for previous treatment with SOF but not if PEG/RBV
c
For those who have failed SOF + RBV with compensated cirrhosis: LDV/SOF + RBV for 24 weeks is recommended
±
Evidence is insufficient if the addition of RBV may benefit subjects with GT3 and cirrhosis. If RBV is not used with
regimen, then baseline RAV testing should be done prior to treatment to rule out the Y93 polymorphism
^ Rarely, genotyping assays may indicate the presence of a mixed infection (e.g., genotypes 1a and 2). Treatment
data for mixed genotypes with direct-acting antivirals are limited. However, in these cases, a pangenotypic regimen is
appropriate.
Ribavirin-containing regimens are absolutely contraindicated in pregnant women and in the male partners of women
who are pregnant. Documented use of two forms of birth control in patients and sex partners for whom a ribavirin-
containing regimen is chosen is required.
Sofosbuvir-containing regimens should not be used in patients with severe renal impairment (GRF < 30 mL/min) or
end stage renal disease requiring dialysis.
Elbasvir/grazoprevir or ombitasvir/paritaprevir/ritonavir + dasubuvir should not be used in patients with moderate to
severe hepatic impairment (CTP and C)
P&T/DUR Review: 5/17(MH); 9/16; 1/16; 5/15; 3/15; 1/15; 9/14; 1/14
Implementation: 6/1/2017; 2/12/16; 4/15; 1/15

Oregon Medicaid PA Criteria 102 July 1, 2017
Hydroxyprogesterone caproate
Goal(s):
To ensure appropriate drug use and limit to patient populations in which hydroxyprogesterone
caproate injection has been shown to be effective and safe.
Length of Authorization:
20 weeks to 6 months (criteria-specific)
Requires PA:
Hydroxyprogesterone caproate injection
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis funded by OHP?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP
3. Is the drug formulation to be used for an
FDA-approved indication?
Message:
Generic formulations of
hydroxyprogesterone caproate are not
approved for prevention of preterm birth
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Is the request for generic
hydroxyprogesterone caproate?
Yes: Go to #5
No: Go to #6
5. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not generally require
a PA. Preferred products are evidence-
based and reviewed for comparative
effectiveness and safety by the P&T
Committee.
Yes: Inform prescriber of
preferred alternatives in
class.
No: Approve for 6
months
6. Is the patient between 16 weeks and 36
weeks 6 days gestation with a singleton
pregnancy?
Yes: Go to #7
No: Pass to RPh. Deny;
medical
appropriateness

Oregon Medicaid PA Criteria 103 July 1, 2017
Approval Criteria
7. Has the patient had a prior history of
preterm delivery before 37 weeks gestation
(spontaneous preterm singleton birth)?
Yes: Go to #8
No: Pass to RPh. Deny;
medical
appropriateness
8. Is treatment being initiated at 16 weeks, 0
days and to 20 weeks, 6 days of gestation?
Yes: Approve through
week 37 of gestation or
delivery, whichever
occurs first (no more
than 20 doses).
No: Pass to RPh. Deny;
medical
appropriateness
P&T/DUR Review: 1/17 (SS); 5/13
Implementation: 4/1/17, 1/1/14

Oregon Medicaid PA Criteria 104 July 1, 2017
Idiopathic Pulmonary Fibrosis (IPF) Agents
Goal:
Restrict use of IPF agent to populations in which the drug has demonstrated efficacy.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Preferred Alternatives:
No preferred alternatives at this time
Approval Criteria
1. Is this request for continuation of
therapy previously approved by the
FFS program (patient has already
been on IPF drug)?
Yes: Go to Renewal Criteria
No: Go to #2
2. Does the patient have a diagnosis of
idiopathic pulmonary fibrosis (ICD-
10 J84112 )?
Yes: Go to #3
No: Pass to RPh. Deny;
medical appropriateness.
3. Is the treatment prescribed by a
pulmonologist?
Yes: Go to #4
No: Pass to RPh. Deny;
medical appropriateness.
4. Does the patient have a forced vital
capacity (FVC) >50%?
Yes: Go to #5
No: Pass to RPh. Deny;
medical appropriateness.
5. Is the patient a current smoker?
Yes: Pass to RPh. Deny; medical
appropriateness.
Efficacy of approved drugs for
IPF may be altered in smokers
due to decreased exposure (see
prescribing information).
No: Go to #6
6. Are pirfenidone and nintedanib
concurrently prescribed in this
patient?
Yes: Pass to RPh. Deny; medical
appropriateness.
Safety and efficacy of
concomitant therapy has not
been established.
No: Approve for up to 12
months.
Renewal Criteria
Is there evidence of disease
progression (defined as ≥10% decline in
percent-predicted FVC) within the
previous 12 months?
Yes: Pass to RPh. Deny; medical
appropriateness.
No: Approve for up to 12
months.
P&T/DUR Review: 7/15 (KS)
Implementation: 8/16, 8/25/15

Oregon Medicaid PA Criteria 105 July 1, 2017
Inhaled Corticosteroids (ICS)
Goals:
Promote use that is consistent with Oregon Asthma Guidelines and the NIH EPR 3 Guidelines on
Asthma. See also:
http://public.health.oregon.gov/DiseasesConditions/ChronicDisease/Asthma/Pages/index.aspx
and
http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/full-report
Step-therapy required prior to coverage for non-preferred ICS products:
o Asthma: inhaled short-acting beta-agonist.
o COPD: short-acting and long-acting bronchodilators (inhaled anticholinergics and beta-
agonists). Preferred short-acting and long-acting bronchodilators do NOT require prior
authorization. See preferred drug list options at http://www.orpdl.org/drugs/.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred ICS products
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 Code
2. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by the
Oregon Pharmacy and Therapeutics (P&T)
Committee.
Yes: Inform prescriber
of covered alternatives
in class.
No: Go to #3
3. Does the patient have a diagnosis of asthma
or reactive airway disease (ICD10 J4520-
J4522, J45901-45998)?
Yes: Go to #7
No: Go to #4

Oregon Medicaid PA Criteria 106 July 1, 2017
Approval Criteria
4. Does the patient have a diagnosis of COPD
(ICD10 J449), chronic bronchitis (ICD10
J410-418, J42, J440-449) and/or
emphysema (ICD10 J439)?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness.
Need a supporting
diagnosis. If prescriber
believes diagnosis is
appropriate, inform
prescriber of the
appeals process for
Medical Director
Review.
5. Does the patient have an active prescription
for an on-demand short-acting
bronchodilator (anticholinergic or beta-
agonist)?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness.
6. Does the patient have an active prescription
for an inhaled long-acting bronchodilator
(anticholinergic or beta-agonist)?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness.
7. Does the patient have an active prescription
for an on-demand short-acting beta-agonist
(SABA) or an alternative rescue medication
for acute asthma exacerbations?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness
P&T Review: 9/16 (KS); 9/15
Implementation: 10/13/16; 10/9/15

Oregon Medicaid PA Criteria 107 July 1, 2017
Initial Pediatric SSRI Antidepressant – Daily Dose Limit
Goals:
Approve only for covered OHP diagnoses.
Limit risk of new-onset of deliberate self-harm thoughts and behaviors, or suicidality associated
with initiation of antidepressant therapy at above recommended doses
Length of Authorization:
Up to 12 months
Requires PA:
Any SSRI in children 0-4 years of age.
Any daily SSRI dose higher than maximum dose in the table below for patients <25 years of
age on date of first antidepressant claim (i.e. no claim for any antidepressant in Specific
Therapeutic Classes H2H, H2S, H2U, H7B, H7C, H7D, H7E, H7J, H8P or H8T in the 102 days
prior)
GSN
SSRI
Age-specific Maximum
Initial Daily Dose
(mg)
Age range (years)
5-9
10-15
16-19
20-24
70991, 46206, 46204, 46203, 46205
citalopram
10
10
20
20
50712, 51642, 51698, 50760
escitalopram
5
10
10
10
46219. 46216, 46217, 47571, 46215,
46214, 46213
fluoxetine
10
10
20
20
46222, 46224. 46225, 46223, 46226,
53387, 53390, 53389, 53388,
paroxetine
(immediate release)
10
10
20
20
46229, 46228, 46227, 46230
sertraline
25
25
50
50
Note: Paroxetine extended release and fluvoxamine are restricted to use in adults
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the patient under 5 years of age?
Yes: Go to #3
No: Go to #4
3. Is the request from a child psychiatrist or
was the regimen developed in consultation
with a child psychiatrist?
Yes: Approve for 12
months
No: Pass to RPH; Deny
Recommend provider
seek a consultation with a
child psychiatrist, such as
the no-cost/same-day
consultation service of
OPAL-K.
www.ohsu.edu/OPALK

Oregon Medicaid PA Criteria 108 July 1, 2017
Approval Criteria
4. Is the patient being treated for funded
diagnosis on the OHP List of Prioritized
Services?
Yes: Go to #5
No: Pass to RPH; Deny,
(Diagnosis not funded by
OHP)
5. Has the patient been treated previously
(within the last 6 months) with a SSRI and is
the dose at or below the maximum
recommended daily dose listed above?
Yes: Approve for 12
months.
No: Go to #6
6. Is the requested dose above the
recommended initial dose listed in the table
above for the patient’s age (i.e. was the
days’ supply entered correctly, is the
patient’s age accurate)?
Yes: Pass to RPh. Go
to #7.
No: Direct Pharmacy to
correct and reprocess
7. Are there clinical circumstances that justify
an increased dose?
Yes: RPh to evaluate
on a case-by-case
basis.
No: Deny for medical
appropriateness
Recommend provider
consider lowering the
initial dose and/or seek a
consultation with a child
psychiatrist, such as the
no-cost/same-day
consultation service of
OPAL-K.
www.ohsu.edu/OPALK
P&T/DUR Review: 9/15 (TW); 7/15; 5/15; 11/14
Implementation: 10/15
Oregon Medicaid PA Criteria 109 July 1, 2017
Insulins
Goal:
Restrict certain insulin products to specific patient populations to ensure appropriate use.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred insulins
All pre-filled insulin pens, cartridges and syringes
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is this an OHP-funded diagnosis?
Yes: Go to #3
No: Pass to RPh.
Deny; not funded by
the OHP
3. Is the request for an insulin pen or cartridge?
Yes: Go to #4
No: Go to #5
4. Is the insulin being administered by the patient
or a non-professional caregiver AND any of the
following criteria apply:
The patient has physical dexterity
problems/vision impairment
The patient is unable to comprehend basic
administration instructions
The patient has a history of dosing errors with
use of vials
The patient is on 40 units or less of insulin per
day
The patient is a child less than 18 years of age?
Yes: Go to #5
No: Pass to RPh.
Deny; medical
appropriateness
5. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are reviewed for comparative
effectiveness and safety by the Oregon
Pharmacy & Therapeutics Committee
Yes: Inform prescriber
of covered alternatives
Approve insulin
pens/cartridges for up
to 12 months (other
preferred products do
not require PA)
No: Approve for up
to 12 months
P&T /DUR Review: 3/16 (KS); 11/15; 9/10
Implementation: 10/13/16; 1/1/11

Oregon Medicaid PA Criteria 110 July 1, 2017
Intranasal Allergy Drugs
Goals:
Restrict use of intranasal allergy inhalers for conditions funded by the OHP and where there is
evidence of benefit.
Treatment for allergic or non-allergic rhinitis is funded by the OHP only if it complicates asthma,
sinusitis or obstructive sleep apnea. Only intranasal corticosteroids have evidence of benefit for
these conditions.
Length of Authorization:
30 days to 6 months
Requires PA:
Preferred intranasal corticosteroids without prior claims evidence of asthma
Non-preferred intranasal corticosteroids
Intranasal antihistamines
Intranasal cromolyn sodium
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Preferred intranasal corticosteroids, preferred second generation antihistamines, and first
generation antihistamines DO NOT require prior authorization.
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the prescribed drug an intranasal
corticosteroid?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP
3. Is the prescribed drug a preferred
product?
Yes: Go to #5
No: Go to #4
4. Will the prescriber consider switching
to a preferred product?
Note:
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy & Therapeutics
Committee.
Yes: Inform prescriber of
preferred alternatives. Go to
#5
No: Go to #5

Oregon Medicaid PA Criteria 111 July 1, 2017
Approval Criteria
5. Does patient have co-morbid
conditions funded by the OHP?
Chronic Sinusitis ( J320-J329)
Acute Sinusitis (J0100; J0110;
J0120; J0130; J0140; J0190)
Sleep Apnea (G4730; G4731;
G4733; G4739)
Yes: Document ICD10
code(s) and approve for up to
6 months for chronic sinusitis
or sleep apnea and approve
for no more than 30 days for
acute sinusitis
No: Go to #6
6. Is there a diagnosis of asthma or
reactive airway disease in the past 1
year (J4520-J4522; J45901-45998)?
Yes: Go to #7
No: Go to #8
7. Is there a claim for an orally inhaled
corticosteroid in the past 90 days?
Note:
Asthma-related outcomes are not
improved by the addition of an intranasal
corticosteroid to an orally inhaled
corticosteroid.
Yes: Pass to RPh. Deny;
medical appropriateness
No: Approve for up to 6
months
8. RPh only: Is the diagnosis funded by
the OHP?
Funded: Deny; medical
appropriateness.
(eg, COPD; Obstructive
Chronic Bronchitis; or other
Chronic Bronchitis [J449;
J40; J410-418; J42; J440-
449]
Use clinical judgment to
APPROVE for 1 month
starting today to allow time
for appeal.
Message: “The request has
been denied because it is
considered medically
inappropriate; however, it has
been APPROVED for 1
month to allow time for
appeal.”
Not Funded: Deny; not
funded by the OHP.
(eg, allergic rhinitis
(J300-J309); chronic
rhinitis (J310-312);
allergic conjunctivitis
(H1045); upper
respiratory infection
(J069); acute
nasopharyngitis
(common cold) (J00);
urticaria (L500-L509);
etc.)
P&T / DUR Review: 11/15 (AG); 7/15; 9/08; 2/06; 9/04; 5/04; 5/02
Implementation: 10/13/16; 1/1/16; 8/25/15; 8/09; 9/06; 3/06; 5/05; 10/04; 8/02

Oregon Medicaid PA Criteria 112 July 1, 2017
Ivabradine (Corlanor
®
)
Goals:
Restrict use of ivabradine to populations in which the drug has demonstrated efficacy.
Encourage use of ACE-inhibitors or angiotensin II receptor blockers (ARBs) with demonstrated
evidence of mortality reduction in heart failure with reduced ejection fraction.
Encourage use of with demonstrated evidence of mortality reduction in heart failure with reduced
ejection fraction.
Length of Authorization:
6 to 12 months
Requires PA:
Ivabradine (Corlanor
®
)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is this a request for continuation of therapy
previously approved by the FFS program
(patient already on ivabradine)?
Yes: Go to Renewal
Criteria
No: Go to #2
2. What diagnosis is being treated?
Record ICD10 code.
3. Does the patient have current
documentation of New York Heart
Association Class II or III heart failure with
reduced ejection fraction less than or equal
to 35% (LVEF ≤ 35%)?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Is the patient in normal sinus rhythm with a
resting heart rate of 70 beats per minute or
greater (≥70 BPM)?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness
5. Has the patient had a previous
hospitalization for heart failure in the past 12
months?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness.

Oregon Medicaid PA Criteria 113 July 1, 2017
Approval Criteria
6. Is the patient currently on a maximally
tolerated dose of carvedilol, sustained-
release metoprolol succinate, or bisoprolol;
and if not, is there a documented intolerance
or contraindication to each of these beta-
blockers?
Note: the above listed beta-blockers have evidence
for mortality reduction in chronic heart failure at
these target doses and are recommended by
national and international heart failure guidelines.
1,2
Carvedilol and metoprolol succinate are preferred
agents on the PDL.
Yes: Go to #7
No: Pass to RPh. Deny;
medical
appropriateness
7. Is the patient currently on a maximally
tolerated dose of an ACE-inhibitor or an
ARB; and if not, is there a documented
intolerance or contraindication to both ACE-
inhibitors and ARBs?
Yes: Go to # 8
No: Pass to RPh. Deny;
medical
appropriateness
8. Is the patient currently on an aldosterone
antagonist; and if not, is there a documented
intolerance or contraindication to therapy
(CrCl < 30 ml/min or potassium ≥ 5.0
mEq/L)?
Note: Aldosterone receptor antagonists
(spironolactone or eplerenone) are recommended in
patients with NYHA class II–IV HF and who have
LVEF of 35% or less, unless contraindicated, to
reduce morbidity and mortality. Patients with NYHA
class II HF should have a history of prior
hospitalization or elevated plasma natriuretic
peptide levels to be considered for aldosterone
receptor antagonists.
Yes: Approve for up to 6
months
No: Pass to RPh. Deny;
medical
appropriateness
Renewal Criteria
1. Is the patient in normal sinus rhythm with no
documented history of atrial fibrillation since
ivabradine was initiated?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness
References:
1. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American
College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol.
2013;62(16):e147-239. doi: 10.1016/j.jacc.2013.05.019.
2. McMurray J, Adamopoulos S, Anker S, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012.
Eur J Heart Fail. 2012;14:803-869. doi:10.1093/eurjhf/hfs105.
P&T / DUR Review: 11/15 (AG)
Implementation: 8/16, 1/1/16

Oregon Medicaid PA Criteria 114 July 1, 2017
Long-acting Beta-agonists (LABA)
Goals:
Promote use that is consistent with Oregon Asthma Guidelines and the NIH EPR 3 Guidelines on
Asthma. See also:
http://public.health.oregon.gov/DiseasesConditions/ChronicDisease/Asthma/Pages/index.aspx
and
http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/full-report
Step-therapy required prior to coverage of non-preferred LABA products:
o Asthma: inhaled corticosteroid and short-acting beta-agonist.
o COPD: inhaled short-acting bronchodilator.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred LABA products
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 Code
2. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform
prescriber of
covered
alternatives in class
No: Go to #3
3. Does the patient have a diagnosis of asthma
or reactive airway disease (ICD10 J4520-
J4522; J45901-45998)?
Yes: Go to #6
No: Go to #4

Oregon Medicaid PA Criteria 115 July 1, 2017
Approval Criteria
4. Does the patient have a diagnosis of COPD
(ICD10 J449), chronic bronchitis (ICD10
J410-418; J42; J440-449) and/or
emphysema (ICD10 J439)?
Yes: Go to #5
No: Pass to RPh. Deny;
medical appropriateness.
Need a supporting diagnosis.
If prescriber believes
diagnosis is appropriate,
inform prescriber of the
appeals process for Medical
Director Review.
5. Does the patient have an active prescription
for an on-demand short-acting
bronchodilator (anticholinergic or beta-
agonist)?
Yes: Approve for
up to 12 months
No: Pass to RPh. Deny;
medical appropriateness.
6. Does the patient have an active prescription
for an on-demand short-acting beta-agonist
(SABA) or an alternative rescue medication
for acute asthma exacerbations?
Yes: Go to #7
No: Pass to RPh. Deny;
medical appropriateness
7. Does the patient have an active prescription
for an inhaled corticosteroid (ICS) or an
alternative asthma controller medication?
Yes: Approve for
up to 12 months
No: Pass to RPh. Deny;
medical appropriateness
P&T Review: 9/16 (KS); 9/15; 5/12; 9/09; 5/09
Implementation: 10/9/15; 8/12; 1/10

Oregon Medicaid PA Criteria 116 July 1, 2017
Long-acting Beta-agonist/Corticosteroid Combination (LABA/ICS)
Goals:
Promote use that is consistent with Oregon Asthma Guidelines and the NIH EPR 3 Guidelines on
Asthma. See also:
http://public.health.oregon.gov/DiseasesConditions/ChronicDisease/Asthma/Pages/index.aspx
and
http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/full-report
Promote use that is consistent with Global Initiative for Chronic Obstructive Lung Disease (GOLD)
Guidelines. See also: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-
management.html
Step-therapy required prior to coverage:
o Asthma: short-acting beta-agonist and inhaled corticosteroid or moderate to severe
persistent asthma.
o COPD: short-acting bronchodilator and previous trial of a long-acting bronchodilator
(inhaled anticholinergic or beta-agonist) or GOLD C/D COPD. Preferred LABA/ICS
products do NOT require prior authorization.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred LABA/ICS products
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 Code
2. Will the provider consider a change to a
preferred product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class
No: Go to #3
3. Does the patient have a diagnosis of asthma
or reactive airway disease (ICD10 J4520-
J4522, J45901-45998)?
Yes: Go to #7
No: Go to #4

Oregon Medicaid PA Criteria 117 July 1, 2017
Approval Criteria
4. Does the patient have a diagnosis of COPD
(ICD10 J449), chronic bronchitis (ICD10
J410-418, J42, J440-449) and/or
emphysema (ICD10 J439)?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness.
Need a supporting
diagnosis. If prescriber
believes diagnosis is
appropriate, inform
prescriber of the
appeals process for
Medical Director
Review.
5. Does the patient have an active prescription
for an on-demand short-acting
bronchodilator (anticholinergic or beta-
agonist)?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness.
6. Is there a documented trial of an inhaled
long-acting bronchodilator (anticholinergic or
beta-agonist), or alternatively has the patient
been assessed with GOLD C/D COPD?
Yes: Approve for up to
12 months. Stop
coverage of all other
LABA and ICS inhalers.
No: Pass to RPh. Deny;
medical
appropriateness.
7. Does the patient have an active prescription
for an on-demand short-acting beta-agonist
(SABA) or an alternative rescue medication
for acute asthma exacerbations?
Yes: Go to #8
No: Pass to RPh. Deny;
medical
appropriateness
8. Is there a documented trial of an inhaled
corticosteroid (ICS) or does the patient have
moderate to severe persistent asthma (Step
3 or higher per NIH EPR 3)?
Yes: Approve for up to
12 months. Stop
coverage of all other ICS
and LABA inhalers.
No: Pass to RPh. Deny;
medical
appropriateness
P&T Review: 9/16 (KS); 11/15; 9/15; 11/14; 11/13; 5/12; 9/09; 2/06
Implementation: 10/13/16; /1/1/16; 1/15; 1/14; 9/12; 1/1

Oregon Medicaid PA Criteria 118 July 1, 2017
Long-acting Muscarinic Antagonist/Long-acting Beta-agonist
Combination (LAMA/LABA)
Goals:
Promote use that is consistent with Oregon Asthma Guidelines and the NIH EPR 3 Guidelines on
Asthma. See also:
http://public.health.oregon.gov/DiseasesConditions/ChronicDisease/Asthma/Pages/index.aspx
and
http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/full-report
Promote COPD therapy that is consistent with Global Initiative for Chronic Obstructive Lung
Disease (GOLD) Guidelines. See also: http://www.goldcopd.org/guidelines-global-strategy-for-
diagnosis-management.html
Step-therapy required prior to coverage:
o COPD: short-acting bronchodilator and previous trial of a long-acting bronchodilator
(inhaled anticholinergic or beta-agonist) or GOLD C/D COPD. Preferred LAMA and LABA
products do NOT require prior authorization.
Length of Authorization:
Up to 12 months
Requires PA:
All LAMA/LABA products
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 Code
2. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of preferred LAMA and
LABA products in each
class
No: Go to #3

Oregon Medicaid PA Criteria 119 July 1, 2017
Approval Criteria
3. Does the patient have a diagnosis of asthma
or reactive airway disease (ICD10 J4520-
J4522, J4540-42, J4550-52, J45901-45998)
without COPD?
Yes: Pass to RPh.
Deny; medical
appropriateness.
Need a supporting
diagnosis. If prescriber
believes diagnosis is
appropriate, inform
prescriber of the appeals
process for Medical
Director Review.
No: Go to #4
4. Does the patient have a diagnosis of COPD
(ICD10 J449), chronic bronchitis (ICD10
J410-418, J42, J440-449) and/or
emphysema (ICD10 J439)?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness.
Need a supporting
diagnosis. If prescriber
believes diagnosis is
appropriate, inform
prescriber of the
appeals process for
Medical Director
Review.
5. Does the patient have an active prescription
for an on-demand short-acting
bronchodilator (anticholinergic or beta-
agonist)?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness.
6. Has the patient been assessed with GOLD
C/D COPD?
Yes: Approve for up to
12 months. Stop
coverage of all other
LAMA and LABA
inhalers.
No: Go to #7
7. Is there a documented trial of a LAMA or
LABA, or alternatively a trial of a fixed dose
combination short-acting anticholinergic with
beta-agonist (SAMA/SABA) (i.e.,
ipratropium/albuterol)?
Yes: Approve for up to
12 months. Stop
coverage of all other
LAMA and LABA
inhalers or scheduled
SAMA/SABA inhalers
(PRN SABA or SAMA
permitted).
No: Pass to RPh. Deny;
medical
appropriateness.
P&T Review: 9/16 (KS); 11/15; 9/15; 11/14; 11/13; 5/12; 9/09; 2/06
Implementation: 10/13/16; 1/1/16; 1/15; 1/14; 9/12; 1/10

Oregon Medicaid PA Criteria 120 July 1, 2017
Lidocaine Patch
Goal(s):
Provide coverage only for funded diagnoses that are supported by the medical literature.
Length of Authorization:
90 days to 12 months (criteria specific)
Requires PA:
Lidocaine Patch
Covered Alternatives
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis an OHP-funded diagnosis with
evidence supporting its use in that condition (refer
to Table 1 for examples).
Yes: Go to # 3
No: Pass to RPh.
Deny; not funded by
the OHP
3. Is this a request for renewal of a previously
approved prior authorization for lidocaine patch?
Yes: Go to Renewal
Criteria
No: Go to # 4
4. Is the prescription for Lidoderm patch greater than
3 patches/day?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Approve for 90
days
Renewal Criteria
1. Does the patient have documented improvement
from lidocaine patch?
Yes: Approve for up
to 12 months
No: Pass to RPh.
Deny for medical
appropriateness.
Table 1. OHP Funded Diagnosis and Evidence Supports Drug Use in Specific Indication
Condition
Lidocaine Patch
Funded
Diabetic Neuropathy
X
Postherpetic
Neuropathy
X
Painful
X

Oregon Medicaid PA Criteria 121 July 1, 2017
Polyneuropathy
Spinal Cord Injury
Pain
Chemotherapy
Induced Neuropathy
Non-funded
Fibromyalgia
P&T Review: 3/17 (DM)
Implementation: 4/1/17

Oregon Medicaid PA Criteria 122 July 1, 2017
Low Dose Quetiapine
Goal(s):
To promote and ensure use of quetiapine that is supported by the medical literature.
To discourage off-label use for insomnia.
Promote the use of non-pharmacologic alternatives for chronic insomnia.
Initiative:
Low dose quetiapine (Seroquel® and Seroquel XR®)
Length of Authorization:
Up to 12 months (criteria-specific)
Requires PA:
Quetiapine (HSN = 14015) doses <150 mg/day
Auto PA approvals for :
o Patients with a claim for a second generation antipsychotic in the last 6 months
o Patients with prior claims evidence of schizophrenia or bipolar disorder
o Prescriptions identified as being written by a mental health provider
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org/drugs/
Zolpidem and benzodiazepine sedatives are available for short-term use (15 doses/30 days)
without PA.
Table 1. Adult (age ≥18 years) FDA-approved Indications for Quetiapine
Bipolar Disorder
F3010; F302; F3160-F3164; F3177-
3178; F319
Major Depressive
Disorder
F314-315; F322-323; F329; F332-333;
F339; F3130
For Seroquel XR® only,
Adjunctive therapy with
antidepressants for Major
Depressive Disorder
Schizophrenia
F205; F209; F2081; F2089
Bipolar Mania
F3010; F339; F3110-F3113; F312
Bipolar Depression
F3130
Table 2. Pediatric FDA-approved indications
Schizophrenia
Adolescents (13-17 years)
Bipolar Mania
Children and Adolescents
(10 to 17 years)
Monotherapy

Oregon Medicaid PA Criteria 123 July 1, 2017
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code. Do not proceed and deny if
diagnosis is not listed in Table 1 or Table 2 above
(medical appropriateness)
2. Is the prescription for quetiapine less than
150 mg/day? (verify days’ supply is
accurate)
Yes: Go to #3
No: Trouble-shoot
claim processing with
the pharmacy.
3. Is planned duration of therapy longer than
90 days?
Yes: Go to #4
No: Approve for
titration up to
maintenance dose (60
days).
4. Is reason for dose <150 mg/day due to any
of the following:
low dose needed due to debilitation
from a medical condition or age;
unable to tolerate higher doses;
stable on current dose; or
impaired drug clearance?
any diagnosis in table 1 or 2 above?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny
for medical
appropriateness.
Note: may approve up
to 6 months to allow
taper.
P&T/DUR Review: 9/15 (KK); 9/10; 5/10
Implementation: 10/15; 1/1/11
Oregon Medicaid PA Criteria 124 July 1, 2017
Milnacipran
Goal(s):
Provide coverage only for funded diagnoses that are supported by the medical literature.
Length of Authorization:
90 days
Requires PA:
Milnacipran
Covered Alternatives
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis an OHP-funded diagnosis with
evidence supporting its use in that condition (see
Table 1 below for examples)?
Yes: Approve for 90
days
No: Pass to RPh.
Deny; not funded by
the OHP
Table 1. OHP Funded or Non-Funded Diagnosis and Evidence Supports Drug Use in Specific
Indication
Condition
Milnacipran
Funded
Diabetic Neuropathy
Postherpetic
Neuropathy
Painful
Polyneuropathy
Spinal Cord Injury
Pain
Chemotherapy
Induced Neuropathy
Non-funded
Fibromyalgia
X
P&T Review: 3/17(DM)
Implementation: 4/1/17

Oregon Medicaid PA Criteria 125 July 1, 2017
Mipomersen and Lomitapide
Goal(s):
To ensure appropriate drug use and limit to patient populations in which mipomersen or lomitapide
has been shown to be effective and safe.
Length of Authorization:
Up to 6 months
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the drug prescribed by or in consultation
with a specialist in lipid disorders?
Yes: Go to #3
No: Pass to RPh. Deny;
medical appropriateness
3. Is the diagnosis homozygous familial
hypercholesterolemia?
Yes: Go to #4
No: Pass to RPh. Deny;
medical appropriateness
4. Has the patient tried and failed or does the
patient have a medical contraindication to
maximum lipid lowering therapy with a
combination of traditional drugs (high-
intensity statin with ezetimibe (see Table 1)?
Yes: Go to #5
No: Pass to RPh. Deny;
medical appropriateness
5. Has the patient failed or are they not
appropriate for LDL-C apheresis; OR is
LDL-C apheresis not available?
Yes: Approve for up
to 12 months
No: Pass to RPh. Deny;
medical appropriateness
Table 1. High-intensity Statins.
High-intensity Statins
(50% LDL-C Reduction)
Atorvastatin 40-80 mg
Rosuvastatin 20-40 mg
Ref. Stone NJ, et al. 2013 ACC/AHA Blood Cholesterol Guideline.
P&T/DUR Review: 11/16 (DM); 5/16; 9/13; 7/13; 5/13
Implementation: 1/1/17; 1/1/14; 11/21/2013

Oregon Medicaid PA Criteria 126 July 1, 2017
Modafinil / Armodafinil
Goal(s):
Limit use to diagnoses where there is sufficient evidence of benefit and uses that are funded by
OHP. Excessive daytime sleepiness related to shift-work is not funded by OHP.
Limit use to safe doses.
Length of Authorization:
Initial approval of 90 days if criteria met; approval of up to 12 months with documented benefit OR
doses above those in Table 2.
Requires PA:
Payment for drug claims for modafinil or armodafinil without previous claims evidence of
narcolepsy or obstructive sleep apnea (ICD10 G47411; G47419; G4730; G4731; G4733; G4739)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Table 1. Funded Indications.
Indication
Modafinil (Provigil™)
Armodafinil (Nuvigil™)
Excessive daytime sleepiness in
narcolepsy
FDA approved for Adults
18 and older
FDA approved for Adults
18 and older
Residual excessive daytime sleepiness in
obstructive sleep apnea patients treated
with CPAP.
FDA approved for Adults
18 and older
FDA approved for Adults
18 and older
Depression augmentation (unipolar or
bipolar)
Not FDA approved;
Low level evidence of
inconsistent benefit
Not FDA approved;
insufficient evidence
Cancer-related fatigue
Not FDA approved;
Low level evidence of
inconsistent benefit
Not FDA approved;
insufficient evidence
Multiple sclerosis-related fatigue
Not FDA approved;
Low level evidence of
inconsistent benefit
Not FDA approved;
insufficient evidence
Drug-related fatigue
Not FDA approved;
insufficient evidence
Not FDA approved;
Excessive daytime sleepiness or fatigue
related to other neurological disorders
(e.g. Parkinson’s Disease, traumatic brain
injury, post-polio syndrome)
Not FDA approved;
insufficient evidence
Not FDA approved;
insufficient evidence
ADHD
Not FDA approved;
Insufficient evidence
Not FDA approved;
insufficient evidence

Oregon Medicaid PA Criteria 127 July 1, 2017
Cognition enhancement for any condition
Not FDA approved;
insufficient evidence
Not FDA approved;
insufficient evidence
Table 2. Maximum Recommended Dose (consistent evidence of benefit with lower doses).
Generic Name
Minimum
Age
Maximum Daily Dose
armodafinil
18 years
250 mg
modafinil
18 years
200 mg
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the patient 18 years of age or older?
Yes: Go to #3
No: Pass to RPh.
Deny; medical
appropriateness
3. Is this a funded diagnosis?
Non-funded diagnoses:
Shift work disorder (ICD10 G4720-
4729; G4750-4769; G478)
Unspecified hypersomnia (ICD10
G4710)
Yes: Go to #4
No: Pass to RPh.
Deny; not funded
by OHP
4. Will prescriber consider a preferred
alternative?
Yes: Inform prescriber of preferred
alternatives (e.g., preferred
methylphenidate)
No: Go to #5
5. Is the request for continuation of
therapy previously approved by the
FFS program?
Yes: Pass to RPh. Go to #13
No: Go to #6
6. Is the prescribed daily dose higher
than recommended in Table 2?
Yes: Pass to RPh. Deny; medical
appropriateness.
No: Go to #7

Oregon Medicaid PA Criteria 128 July 1, 2017
Approval Criteria
7. Is diagnosis narcolepsy or obstructive
sleep apnea (ICD10 G47411; G47419;
G4730; G4731; G4733; G4739) AND is
the drug prescribed by, or in
consultation with, a sleep specialist or
neurologist?
Yes: Approve for 90 days and
inform prescriber further approval
will require documented evidence
of clinical benefit.
No: Go to #8
8. Is the request for armodafinil?
Yes: Pass to RPh. Deny; medical
appropriateness.
There is insufficient evidence for
off-label use.
No: Go to #9
9. Is the diagnosis unipolar or bipolar
depression?
Yes: Approve for 90 days and
inform prescriber further approval
will require documented evidence
of clinical benefit.
No: Go to #10
10. Is the diagnosis MS or cancer-related
fatigue?
Note: Methylphenidate is
recommended first-line for cancer.
Yes: Inform prescriber of first-line
options available without PA.
May approve for 90 days and
inform prescriber further approval
will require documented evidence
of clinical benefit.
No: Go to #11
11. Is the diagnosis ADHD?
Yes: Pass to RPh. Deny; medical
appropriateness.
There is insufficient evidence for
benefit for ADHD. See available
options at www.orpdl.org/drugs/
No: Go to #12
12. All other diagnoses must be evaluated as to the OHP-funding level and evidence for clinical
benefit.
Evidence supporting treatment for excessive daytime sleepiness or fatigue as a result of other
conditions is currently insufficient and should be denied for “medical appropriateness”.
Evidence to support cognition enhancement is insufficient and should be denied for “medical
appropriateness”.
If new evidence is provided by the prescriber, please forward request to Oregon DMAP for
consideration and potential modification of current PA criteria.
Oregon Medicaid PA Criteria 129 July 1, 2017
Approval Criteria
13. Continuation of therapy requires submission of documented evidence of clinical benefit and
tolerability (faxed copy or equivalent). The same clinical measure (eg, Epworth score, Brief
Fatigue Inventory, or other validated measure) used to diagnose fatigue or depression is
recommended to document clinical benefit.
Approve up to 12 months with chart documentation of positive response.
Deny for “medical appropriateness” in absence of documented benefit.
P&T Review: 03/16; 09/15
Implementation: 8/16, 1/1/16

Oregon Medicaid PA Criteria 130 July 1, 2017
Monoclonal Antibodies for Severe Asthma
Goal(s):
Restrict use of monoclonal antibodies to patients with severe asthma requiring chronic systemic
corticosteroid use or with history of asthma exacerbations in the past year that required an
Emergency Department visit or hospitalization.
Restrict use for conditions not funded by the OHP (e.g., chronic urticaria).
Length of Authorization:
Up to 12 months
Requires PA:
Omalizumab
Mepolizumab
Reslizumab
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Table 1. Maximum Adult Doses for Inhaled Corticosteroids.
High Dose Corticosteroids:
Maximum Dose
Qvar (beclomethasone)
320 mcg BID
Pulmicort Flexhaler (budesonide)
720 mcg BID
Alvesco (ciclesonide)
320 mcg BID
Aerospan (flunisolide)
320 mcg BID
Arnuity Ellipta (fluticasone furoate)
200 mcg daily
Flovent HFA (fluticasone propionate)
880 mcg BID
Flovent Diskus (fluticasone propionate)
1000 mcg BID
Asmanex Twisthaler (mometasone)
440 mcg BID
Asmanex HFA (mometasone)
400 mcg BID
High Dose Corticosteroid / Long-acting Beta-agonists
Maximum Dose
Symbicort (budesonide/formoterol)
320/9 mcg BID
Advair Diskus (fluticasone/salmeterol)
500/50 mcg BID
Advair HFA (fluticasone/salmeterol)
460/42 mcg BID
Breo Ellipta (fluticasone/vilanterol)
200/25 mcg daily
Dulera (mometasone/formoterol)
400/10 mcg BID
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the request for continuation of therapy
previously approved by the FFS program?
Yes: Go to Renewal
Criteria
No: Go to #3
3. Is the claim for reslizumab in a patient under
18 years of age?
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Go to #4
Oregon Medicaid PA Criteria 131 July 1, 2017
Approval Criteria
4. Is the claim for mepolizumab in a patient
under 12 years of age?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #5
5. Is the diagnosis an OHP-funded diagnosis?
Note: chronic urticaria is not an OHP-funded
condition
Yes: Go to #6
No: Pass to RPh. Deny;
not funded by the OHP.
6. Is the prescriber a pulmonologist or an
allergist who specializes in management of
severe asthma?
Yes: Go to #7
No: Pass to RPh. Deny;
medical
appropriateness.
7. Has the patient required at least 2
hospitalizations or ED visits in the past 12
months while receiving a maximally-dosed
inhaled corticosteroid (Table 1) AND 2
additional controller drugs (i.e., long-acting
inhaled beta-agonist, montelukast,
zafirlukast, aminophylline, theophylline)?
Yes: Go to #8
Document number of
hospitalizations or ED
visits for asthma
exacerbation in past 12
months: __________.
This is the baseline
value to compare to in
renewal criteria.
No: Pass to RPh. Deny;
medical
appropriateness.
8. Has the patient been adherent to current
asthma therapy in the past 12 months?
Yes: Go to #9
No: Pass to RPh. Deny;
medical
appropriateness.
9. Is the patient currently receiving another
monoclonal antibody for asthma (e.g.,
omalizumab, mepolizumab or reslizumab)?
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Go to #10
10. If the claim is for omalizumab, can the
prescriber provide documentation of allergic
IgE-mediated asthma diagnosis, confirmed
by a positive skin test or in vitro reactivity to
perennial allergen?
Yes: Approve once
every 2-4 weeks for up
to 12 months.
Document test and
result:__________
No: Go to #11
11. If the claim is for mepolizumab or
reslizumab, can the prescriber provide
documentation of eosinophilic phenotype,
confirmed by blood eosinophil count ≥300
cells/μL in the past 12 months?
Yes: Approve once
every 4 weeks for up to
12 months.
Document eosinophil
count
(date):__________
No: Pass to RPh. Deny;
medical
appropriateness.
Oregon Medicaid PA Criteria 132 July 1, 2017
Renewal Criteria
1. Is the patient currently taking a maximally-
dosed inhaled corticosteroid and 2
additional controller drugs (i.e., long-acting
inhaled beta-agonist, montelukast,
zafirlukast, aminophylline, theophylline)?
Yes: Go to #2
No: Pass to RPh. Deny;
medical
appropriateness.
2. Has the number of ED visits or
hospitalizations in the last 12 months been
reduced from baseline, or has the patient
reduced their systemic corticosteroid dose
by ≥50% compared to baseline?
Yes: Approve for up to
12 months.
No: Pass to RPh. Deny;
medical
appropriateness.
P&T Review: 7/16
Implementation: 8/16

Oregon Medicaid PA Criteria 133 July 1, 2017
Oral Multiple Sclerosis Drugs
Goal(s):
Promote safe and effective use of oral disease-modifying multiple sclerosis drugs
Promote use of preferred multiple sclerosis drugs.
Length of Authorization:
Up to 12 months
Requires PA:
Fingolimod
Teriflunomide
Dimethyl Fumarate
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Does the patient have a diagnosis of
relapsing remitting multiple sclerosis?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP.
See Guideline Note 95
in the Prioritized List of
Health Services.
3. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are reviewed for
comparative effectiveness and safety by
the Pharmacy and Therapeutics
Committee and do not require PA.
Yes: Inform prescriber
of covered alternatives
in class.
No: Go to #4
4. Has the patient failed or cannot tolerate a
trial of interferon beta 1a or interferon beta
1b, and glatiramer?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness.
5. Is the medication being prescribed by or in
consultation with a neurologist?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness.
6. Is the patient on concurrent treatment with a
disease modifying drug (i.e. interferon beta
1B, glatiramer acetate, interferon beta 1A,
natalizumab, mitoxantrone)?
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Go to #7
7. Is the prescription for teriflunomide?
Yes: Go to #8
No: Go to #10

Oregon Medicaid PA Criteria 134 July 1, 2017
Approval Criteria
8. Is the patient of childbearing potential?
Yes: Go to #9
No: Approve for up to 1
year.
9. Is the patient currently on a documented use
of reliable contraception and is there
documentation of a negative pregnancy test
prior to initiation of teriflunomide?
Yes: Approve for up to 1
year.
No: Pass to RPh. Deny;
medical
appropriateness.
10. Is the prescription fingolimod?
Yes: Go to #11
No: Go to #14
11. Does the patient have evidence of macular
edema?
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Go to #12
12. Does the patient have preexisting cardiac
disease, risk factors for bradycardia, or is on
anti-arrhythmic, beta-blockers, or calcium
channel blockers?
Yes: Go to #13
No: Approve up to 1
year.
13. Has the patient had a cardiology
consultation before initiation (see clinical
notes)?
Yes: Approve up to 1
year.
No: Pass to RPh. Deny;
medical
appropriateness.
14. Is the prescription for dimethyl fumarate?
Yes: Go to # 15
No: Pass to RPh. Deny;
medical
appropriateness.
15. Does patient have a baseline CBC with
lymphocyte count greater than 500/µL?
Yes: Approve for up to 1
year
No: Pass to RPh. Deny;
medical
appropriateness.
Fingolimod Clinical Notes:
Because of bradycardia and atrioventricular conduction, patients must be observed for 6 hours after initial dose in a
clinically appropriate area.
Patients on antiarrhythmics, beta-blockers or calcium channel blockers or with risk factors for bradycardia (h/o MI,
age >70 yrs., electrolyte disorder, hypothyroidism) may be more prone to development of symptomatic bradycardia
and should be initiated on fingolimod with caution. A cardiology evaluation should be performed before considering
treatment.
Injectable disease modifying treatments remain first-line agents in MS therapy.
An ophthalmology evaluation should be repeated 3-4 months after fingolimod initiation with subsequent evaluations
based on clinical symptoms.
Teriflunomide Clinical Notes:
Before starting teriflunomide, screen patients for latent tuberculosis infection with a TB skin test, exclude pregnancy,
confirm use of reliable contraception in women of childbearing potential, check blood pressure, and obtain a
complete blood cell count within the 6 months prior to starting therapy. Instruct patients to report symptoms of
infection and obtain serum transaminase and bilirubin levels within the 6 months prior to starting therapy.
After starting feriflunomide, monitor ALT levels at least monthly for 6 months. Consider additional ALT monitoring
when feriflunomide is given with other potentially hepatotoxic drugs. Consider stopping feriflunomide if serum
transaminase levels increase (>3-times the ULN). Monitor serum transaminase and bilirubin particularly in patients

Oregon Medicaid PA Criteria 135 July 1, 2017
who develop symptoms suggestive of hepatic dysfunction. Discontinue teriflunomide and start accelerated
elimination in those with suspected teriflunomide-induced liver injury and monitor liver tests weekly until normalized.
Check blood pressure periodically and manage hypertension. Check serum potassium level in teriflunomide-treated
patients with hyperkalemia symptoms or acute renal failure. Monitor for signs and symptoms of infection.
Monitor for hematologic toxicity when switching from teriflunomide to another agent with a known potential for
hematologic suppression because systemic exposure to both agents will overlap.
Dimethyl Fumarate Clinical Notes:
Dimethyl fumarate may decrease a patient’s white blood cell count. In the clinical trials the mean lymphocyte
counts decreased by approximately 30% during the first year of treatment with dimethyl fumarate and then
remained stable. The incidence of infections (60% vs. 58%) and serious infections (2% vs. 2%) was similar in
patients treated with dimethyl fumurate or placebo, respectively. There was no increased incidence of serious
infections observed in patients with lymphocyte counts <0.8 x10
3
cells/mm
3
. A transient increase in mean
eosinophil counts was seen during the first 2 months of therapy.
Dimethyl fumarate should be held if the WBC falls below 2 x10
3
cells/mm
3
or the lymphocyte count is below 0.5
x10
3
cells/mm
3
and permanently discontinued if the WBC did not increase to over 2 x10
3
cells/mm
3
or
lymphocyte count increased to over 0.5 x10
3
cells/mm
3
after 4 weeks of withholding therapy.
Patients should have a CBC with differential monitored on a quarterly basis
P&T/DUR Review: 11/16 (DM); 9/15; 9/13; 5/13; 3/12
Implementation: TBD; 1/1/14; 6/21/2012

Oregon Medicaid PA Criteria 136 July 1, 2017
Multivitamins
Goals:
Restrict use for documented nutritional deficiency or diagnosis associated with nutritional
deficiency (e.g., Cystic Fibrosis)
Prenatal and pediatric multivitamins are not subject to this policy.
Length of Authorization:
Up to 12 months
Requires PA:
All multivitamins in HIC3 = C6B, C6G, C6H, C6I, C6Z
Covered Alternatives:
Upon PA approval, only vitamins generically equivalent to those listed below will be covered:
GSN Generic Name Example Brand
002532 MULTIVITAMIN DAILY VITE OR TAB-A-VITE
039744 MULTIVITS, TH W-FE, OTHER MIN THEREMS-M
002523 MULTIVITAMINS, THERAPEUTIC THEREMS
064732 MULTIVITAMIN/ IRON/ FOLIC ACID CEROVITE ADVANCED FORMULA
048094 MULTIVITAMIN W-MINERALS/ LUTEIN CEROVITE SENIOR
002064 VITAMIN B COMPLEX VITAMIN B COMPLEX
058801 MULTIVITS-MIN/ FA/ LYCOPENE/ LUT CERTAVITE SENIOR-ANTIOXIDANT
047608 FOLIC ACID/ VITAMIN B COMP W-C NEPHRO-VITE
022707 BETA-CAROTENE (A) W-C & E/MIN PROSIGHT
061112 VIT A, C & E/ LUTEIN/ MINERALS OCUVITE WITH LUTEIN
066980 MULTIVAMIN/ FA/ ZINC ASCORBATE SOURCECF
067025 PEDIATRIC MULTIVIT #22/ FA/ ZINC SOURCECF
058068 MULTIVITAMIN/ ZINC GLUCONATE SOURCECF
068128 PEDIATRIC MULTIVIT #32/ FA/ ZINC AKEDAMINS
061991 PEDI MULTIVIT #40/ PHYTONADIONE AQUADEKS
066852 MULTIVITS & MINS/ FA/ COENZYME Q10 AQUADEKS
068035 MULTIVITS & MINS/ FA/ COENZYME Q10 AQUADEKS
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is this an OHP-funded diagnosis?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP

Oregon Medicaid PA Criteria 137 July 1, 2017
Approval Criteria
3. Does the patient have a documented
nutrient deficiency
OR
Does the patient have an increased
nutritional need resulting from severe
trauma (e.g., severe burn, major bone
fracture, etc.)
OR
Does the patient have a diagnosis resulting
in malabsorption (e.g., Crohn’s disease,
Cystic Fibrosis, bowel resection or removal,
short gut syndrome, gastric bypass, renal
dialysis, dysphagia, achalasia, etc.)
OR
Does the patient have a diagnosis that
requires increased vitamin or mineral
intake?
Yes: Approve up to 1
year
No: Pass to RPh. Deny;
medical
appropriateness.
P&T Review: 3/16 (MH/KK); 3/14
Implementation: 5/1/16, 4/1/2014

Oregon Medicaid PA Criteria 138 July 1, 2017
New Drug Policy
Goal:
Restrict coverage of selected new drugs until the Oregon Pharmacy & Therapeutics Committee
can review the drug for appropriate coverage.
Length of Authorization:
Up to 6 months
Requires PA:
A new drug, identified by the reviewing pharmacist during the weekly claim processing drug file
load, in a class where existing prior authorization policies exist or that is used for a non-funded
condition on the Oregon Health Plan (OHP) List of prioritized services.
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the drug being used to treat an OHP-
funded condition?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP.
3. Pass to RPh. The prescriber must provide documentation of therapeutic failure, adverse event, or
contraindication alternative drugs approved by FDA for the funded condition. Otherwise, the
prescriber must provide medical literature supporting use for the funded condition. RPh may use
clinical judgement to approve drug for up to 6 months or deny request based on documentation
provided by prescriber.
P&T / DUR Review: 11/15 (AG); 12/09
Implementation: 1/1/16; 1/1/10
Oregon Medicaid PA Criteria 139 July 1, 2017
Nusinersen
Goal(s):
Approve nusinersen for funded OHP conditions supported by evidence of benefit (e.g. Spinal
Muscular Atrophy)
Length of Authorization:
Up to 12 months
Requires PA:
Nusinersen
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code. Go to # 2
2. Is this a request for continuation of therapy?
Yes: Deny; Refer
request for renewal of
therapy to DMAP
medical director for
review.
No: Go to #3
3. Does the patient have Spinal Muscular
Atrophy (SMA) documented by genetic
testing?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness.
4. Is the drug being prescribed by a neurologist
or a provider with experience treating spinal
muscular atrophy?
Yes: Approve up to 12
months
No: Pass to RPh. Deny;
medical
appropriateness.
P&T Review: 3/17 (DM)
Implementation 4/1/17

Oregon Medicaid PA Criteria 140 July 1, 2017
Nutritional Supplements (Oral Administration Only)
Goals:
Restrict use to patients unable to take food orally in sufficient quantity to maintain adequate
weight.
Requires ANNUAL nutritional assessment for continued use.
Use restriction consistent with DMAP EP/IV rules at:
http://www.oregon.gov/oha/healthplan/Pages/home-epiv.aspx
These products are NOT federally rebate-able; Oregon waives the rebate requirement for this class.
Note:
Nutritional formulas, when administered enterally (G-tube) are no longer available through the
point-of-sale system.
Service providers should use the CMS 1500 form and mail to DMAP, P.O. Box 14955, Salem,
Oregon, 97309 or the 837P electronic claim form and not bill through POS.
When billed correctly with HCPCS codes for enterally given supplements, enterally
administered nutritional formulas do not require prior authorization (PA). However, the
equipment do require a PA (i.e., pump).
Providers can be referred to 800-642-8635 or 503-945-6821 for enteral equipment PAs
For complete information on how to file a claim, go to:
http://www.oregon.gov/oha/healthplan/Pages/home-epiv.aspx
Length of Authorization:
Up to 12 months
Note:
Criteria is divided into: 1) Patients age 6 years or older
2) Patients under 6 years of age
Not Covered:
Supplements such as acidophilis, Chlorophyll, Coenzyme Q10 are not covered and should not be
approved.
Requires PA:
All supplemental nutrition products in HIC3 = C5C, C5F, C5G, C5U, C5B
(nutritional bars, liquids, packets, powders, wafers such as Ensure, Ensure Plus, Nepro,
Pediasure, Promod).
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/

Oregon Medicaid PA Criteria 141 July 1, 2017
Patients 6 years and older:
Document:
Name of product being requested
Physician name
Quantity/Length of therapy being requested
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is product requested a supplement or herbal
product without an FDA indication?
Yes: Pass to RPh.
Deny; medical
appropriateness)
No: Go to #3
3. Is the product to be administered by enteral tube
feeding (e.g., G-tube)?
Yes: Go to #10
No: Go to #4
4. All indications need to be evaluated as to
whether they are funded conditions under the
OHP.
Funded: Go to #5
Not Funded: Pass to
RPh. Deny; not
funded by the OHP.
5. Is this request for continuation of therapy
previously approved by the FFS program?
Yes: Go to #6
No: Go to #7
6. Has there been an annual assessment by a
physician for continued use of nutritional
supplementation?
Document assessment date.
Yes: Approve up to 1
year
No: Request
documentation of
assessment.
Without
documentation, pass
to RPh. Deny;
medical
appropriateness.
7. Patient must have a nutritional deficiency
identified by one of the following:
Recent (within 1 year) Registered
Dietician assessment indicating adequate
intake is not obtainable through
regular/liquefied or pureed foods
(supplement cannot be approved for
convenience of patient or caregiver);
OR
Recent serum protein level <6 g/dL?
Yes: Go to #9
No: Go to #8

Oregon Medicaid PA Criteria 142 July 1, 2017
Approval Criteria
8. Does the patient have a prolonged history (>1
year) of malnutrition and cachexia OR reside in
a long-term care facility or nursing home?
Document:
Residence
Current body weight
Ideal body weight
Yes: Go to #9
No: Request
documentation.
Without
documentation, pass
to RPh. Deny;
medical
appropriateness.
9. Does the patient have a recent unplanned
weight loss of at least 10%, plus one of the
following:
increased metabolic need resulting from
severe trauma (e.g., severe burn, major
bone fracture, etc.);
OR
malabsorption (e.g., Crohn’s Disease,
Cystic Fibrosis, bowel resection/removal,
Short Gut Syndrome, gastric bypass,
hemodialysis, dysphagia, achalasia, etc.);
OR
diagnosis that requires additional calories
and/or protein intake (e.g., malignancy,
AIDS, pulmonary insufficiency, MS, ALS,
Parkinson’s, Cerebral Palsy, Alzheimer’s,
etc.)?
Yes: Approve for up to
1 year
No: Request
documentation.
Without
documentation, pass
to RPh. Deny;
medical
appropriateness.
10. Is this request for continuation of therapy previously approved by the FFS program?
Yes: Approve for 1 month and reply:
Nutritional formulas, when administered by enteral tube, are no longer available through the
point-of-sale (POS) system. For future use, service providers should use the CMS form
1500 or the 837P electronic claim form and not bill through POS. A 1-month approval has
been given to accommodate the transition.
Go to: http://www.oregon.gov/oha/healthplan/Pages/home-epiv.aspx
No: Enter an Informational PA and reply: Nutritional formulas, when administered by
enteral tube, are no longer available through the point-of-sale (POS) system. For future
use, service providers should use the CMS form 1500 or the 837P electronic claim form
and not bill through POS. When billed using a HCPCS code, enterally administered
nutritional formulas do not require a prior authorization (PA). However, the equipment does
require a PA. Providers can be referred to 800-642-8635 or 503-945-6821 for enteral
equipment PAs.
For complete information of how to file a claim, go to:
http://www.oregon.gov/oha/healthplan/Pages/home-epiv.aspx

Oregon Medicaid PA Criteria 143 July 1, 2017
Patients under 6 years of age
Document:
Name of product requested
Physician name
Quantity/Length of therapy requested
Approval Criteria
1. What diagnosis is being treated?
Record the ICD10 code
2. Is the product to be administered by enteral tube
feeding (e.g., G-tube)?
Yes: Go to #9
No: Go to #3
3. All indications need to be evaluated as to
whether they are funded conditions under the
OHP.
Funded: Go to #4
Not Funded: Pass
to RPh. Deny; not
funded by the OHP.
4. Is this request for continuation of therapy
previously approved by the FFS program?
Yes: Go to #5
No: Go to #6
5. Has there been an annual assessment by a
physician for continued use of nutritional
supplementation?
Document assessment date.
Yes: Approve up to 1
year
No: Request
documentation.
Without
documentation,
pass to RPh. Deny;
medical
appropriateness.
6. Is the diagnosis failure-to-thrive (FTT)?
Yes: Approve for up to
1 year
No: Go to #7
7. Does the patient have one of the following:
increased metabolic need resulting from
severe trauma (e.g., severe burn, major
bone fracture, etc.);
OR
malabsorption (e.g., Crohn’s Disease,
Cystic Fibrosis, bowel resection/removal,
Short Gut Syndrome, hemodialysis,
dysphagia, achalasia, etc.);
OR
diagnosis that requires additional calories
and/or protein intake (e.g., malignancy,
AIDS, pulmonary insufficiency, Cerebral
Palsy, etc.)?
Yes: Approve for up to
1 year
No: Go to #8

Oregon Medicaid PA Criteria 144 July 1, 2017
8. Patient must have a nutritional deficiency
identified by one of the following:
Recent (within 1 year) Registered
Dietician assessment indicating adequate
intake is not obtainable through
regular/liquefied or pureed foods
(supplement cannot be approved for
convenience of patient or caregiver);
OR
Recent serum protein level <6 g/dL?
Yes: Approve for up to
1 year
No: Request
documentation.
Without
documentation,
pass to RPh. Deny;
medical
appropriateness.
9. Is this request for continuation of therapy previously approved by the FFS program?
Yes: Approve for 1 month and reply:
Nutritional formulas, when administered by enteral tube, are no longer available through
the point-of-sale (POS) system. For future use, service providers should use the CMS
form 1500 or the 837P electronic claim form and not bill through POS. A 1-month approval
has been given to accommodate the transition.
Go to: http://www.oregon.gov/oha/healthplan/Pages/home-epiv.aspx
No: Enter an Informational PA and reply: Nutritional formulas, when administered by
enteral tube, are no longer available through the point-of-sale (POS) system. For future
use, service providers should use the CMS form 1500 or the 837P electronic claim form
and not bill through POS. When billed using a HCPCS code, enterally administered
nutritional formulas do not require a prior authorization (PA). However, the equipment
does require a PA. Providers can be referred to 800-642-8635 or 503-945-6821 for
enteral equipment PAs.
For complete information of how to file a claim, go to:
http://www.oregon.gov/oha/healthplan/Pages/home-epiv.aspx
Note: Normal Serum Protein 6-8 g/dL
Normal albumin range 3.5-5.5 g/dL
P&T Review: 11/14
Implementation: 10/13/16; 1/1/15; 6/22/07; 9/1/06; 4/1/03

Oregon Medicaid PA Criteria 145 July 1, 2017
Obeticholic Acid (Ocaliva
®
)
Goal(s):
Encourage use of ursodiol or ursodeoxycholic acid which has demonstrated decrease disease
progression and increase time to transplantation.
Restrict use to populations for which obeticholic acid has demonstrated efficacy.
Length of Authorization:
Up to 12 months
Requires PA:
Obeticholic acid
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is this request for continuation of therapy
previously approved by the FFS program
(patient has already been on obeticholic
acid)?
Yes: Go to Renewal
Criteria
No: Go to #3
3. Is the treatment for primary biliary
cholangitis?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Does the patient have no evidence of
complications from cirrhosis or hepatic
decompensation (e.g., MELD score less
than 15; not awaiting transplant; no portal
hypertension; or no hepatorenal syndrome)?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness
5. Is the total bilirubin level less than 2-times
the upper limit of normal (ULN)?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness
6. Does patient have a documented
intolerance or contraindication to ursodiol?
Yes: Document
symptoms of intolerance
or contraindication and
approve for up to 12
months
No: Go to #7
Oregon Medicaid PA Criteria 146 July 1, 2017
Approval Criteria
7. Has patient had a 12-month trial of ursodiol
with inadequate response to therapy (ALP
≥1.67-times the ULN or total bilirubin greater
than the ULN)?
Yes: Document baseline
ALP and total bilirubin
level and appprove for
up to 12 months
ALP:___________
units/L
Total Bilirubin
_________ mg/dL
No: Pass to RPh. Deny;
medical
appropriateness
Renewal Criteria
1. Is there evidence of improvement of primary
biliary cholangitis, defined as:
a. ALP <1.67-times the ULN; AND
b. Decrease of ALP >15% from
baseline: AND
c. Normal total bilirubin level?
Yes: Document ALP
and total bilirubin level
and approve for up to
12 months
ALP:___________
units/L
Total Bilirubin
_________ mg/dL
No: Pass to RPh. Deny;
medical
appropriateness
P&T / DUR Review: 01/17 (SS)
Implementation: 4/1/17

Oregon Medicaid PA Criteria 147 July 1, 2017
Ocular Vascular Endothelial Growth Factors
Goal(s):
Promote use of preferred drugs and ensure that non-preferred drugs are used appropriately for
OHP-funded conditions
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is this an OHP-funded diagnosis?
Yes: Go to #3
No: Go to #4
3. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require a PA.
Preferred products are evidence-based and
reviewed for comparative effectiveness and
safety by the P&T Committee.
Yes: Inform prescriber of
covered alternatives in
class.
No: Approve for 12
months, or for length of
the prescription,
whichever is less
4. RPh only: All other indications need to be evaluated as to whether they are funded or contribute to
a funded diagnosis on the OHP prioritized list.
If funded and clinic provides supporting literature: Approve for 12 months, or for length of the
prescription, whichever is less.
If not funded: Deny; not funded by the OHP.
P&T / DUR Review: 3/17 (SS)
Implementation: TBD

Oregon Medicaid PA Criteria 148 July 1, 2017
Omega-3 Fatty Acids
Goal(s):
Restrict use of omega-3 fatty acids to patients at increased risk for pancreatitis.
Length of Authorization:
Up to 12 months
Requires PA:
Omega-3-Acid Ethyl Esters (Lovaza
®
)
Icosapent Ethyl (Vascepa
®
)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis an OHP funded diagnosis?
Yes: Go to #3
No: Pass to RPh.
Deny; not funded by
the OHP
3. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require PA.
Preferred products are reviewed for
comparative effectiveness and safety by
the Oregon Pharmacy and Therapeutics
Committee.
Yes: Inform prescriber of
covered alternatives in
class.
No: Go to #4
4. Does the patient have clinically diagnosed
hypertriglyceridemia with triglyceride levels
≥ 500 mg/dL?
Yes: Go to #5
No: Pass to RPh.
Deny; medical
appropriateness.
5. Has the patient failed or have a
contraindication to an adequate trial (at least
8 weeks) of a fibric acid derivative
(fenofibrate or gemfibrozil) at a maximum
tolerable dose (as seen in dosing table
below); OR
Is the patient taking a statin and unable to
take a fibric acid derivative due to an
increased risk of myopathy?
Yes: Approve up to 1
year.
No: Pass to RPh.
Deny; medical
appropriateness.
Recommend trial of
other agent(s).

Oregon Medicaid PA Criteria 149 July 1, 2017
Table 1: Dosing of Fenofibrate and Derivatives for Hypertriglyceridemia.
Trade Name (generic)
Recommended dose
Maximum dose
Antara (fenofibrate capsules)
43-130 mg once daily
130 mg once daily
Fenoglide (fenofibrate tablet)
40-120 once daily
120 mg once daily
Fibricor (fenofibrate tablet)
25-105 mg once daily
105 mg once daily
Lipofen (fenofibrate capsule)
50-150 mg once daily
150 mg once daily
Lofibra (fenofibrate capsule)
67-200 mg once daily
200 mg once daily
Lofibra (fenofibrate tablet)
54-160 mg once daily
160 mg once daily
Lopid (gemfibrozil tablet)
600 mg twice daily
600 mg twice daily
Tricor (fenofibrate tablet)
48-145 mg once daily
145 mg once daily
Triglide (fenofibrate tablet)
50-160 mg once daily
160 mg once daily
Trilipix (fenofibrate DR capsule)
45-135 mg once daily
135 mg once daily
P&T/DUR Review: 11/16 (DM); 3/14
Implementation: 1/1/17; 5/1/14
Oregon Medicaid PA Criteria 150 July 1, 2017
Opioid Analgesics
Goals:
Restrict use of opioid analgesics to OHP-funded conditions with documented sustained
improvement in pain and function and with routine monitoring for opioid misuse and abuse.
Promote the safe use of opioid analgesics by restricting use of high doses that have not
demonstrated improved benefit and are associated with greater risk for accidental opioid overdose
and death.
Limit the use of non-preferred opioid analgesic products.
Length of Authorization:
3 to 12 months (criteria-specific)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Requires a PA:
All non-preferred opioids and opioid combination products.
Any opioid listed in Table 1 or opioid combination product that contains an opioid listed in
Table 1 that exceeds 90 morphine milligram equivalents (MME) per day.
Any opioid product listed in Table 2 that exceeds quantity limits.
Note:
Preferred opioid products that do not exceed 90 MME per day are exempt from this PA.
Patients on palliative care with a terminal diagnosis or with cancer-related pain (ICD10 C6900-
C799; C800-C802) are exempt from this PA.
This PA does not apply to pediatric use of codeine products, which is subject to separate
clinical PA criteria.
Table 1. Daily Dose Threshold (90 MME/day) of Opioid Products.
Opioid
90 MME/day
Notes
Codeine
600 mg
Codeine is not recommended for pediatric use; codeine is a prodrug of morphine
and is subject to different rates of metabolism placing certain populations at risk
for overdose.)
Fentanyl
(transdermal
patch)
37.5 mcg/hr
Use only in opioid-tolerant patients who have been taking ≥60 MME daily for a ≥1
week. Deaths due to a fatal overdose of fentanyl have occurred when pets,
children and adults were accidentally exposed to fentanyl transdermal patch.
Strict adherence to the recommended handling and disposal instructions is of the
utmost importance to prevent accidental exposure.)
Hydrocodone
90 mg
Hydromorphone
22.5 mg
Morphine
90 mg
Oxycodone
60 mg
Oxymorphone
30 mg
Tapentadol
225 mg
Tramadol ER
300 mg
300 mg/day is max dose and is not equivalent to 90 MME/day.
Tramadol IR
400 mg
300 mg/day is max dose and is not equivalent to 90 MME/day.
Methadone*
20 mg
*DO NOT USE unless very familiar with the complex pharmacokinetic and
pharmacodynamics properties of methadone. Methadone exhibits a non-
linear relationship due to its long half-life and accumulates with chronic dosing.
Methadone also has complex interactions with several other drugs. The dose
should not be increased more frequently than once every 7 days. Methadone is
associated with an increased incidence of prolonged QTc interval, torsades de
pointe and sudden cardiac death.
Oregon Medicaid PA Criteria 151 July 1, 2017
Abbreviations: ER = extended-release or sustained-release formulation(s); IR = immediate-release formulation(s); MME = morphine
milligram equivalent.
Table 2. Specific Opioid Products Subject to Quantity Limits per FDA-approved Labeling.
Drug Product
Quantity Limit
Drug Product
Quantity Limit
Drug Product
Quantity Limit
AVINZA
1 dose/day
HYSINGLA
1 dose/day
OXYCONTIN
2 doses/day
BELBUCA
2 doses/day
KADIAN
2 doses/day
TROXYCA ER
2 doses/day
BUTRANS
1 patch/7 days
MORPHABOND
2 doses/day
ULTRAM ER
1 dose/day
CONZIP
1 dose/day
MS CONTIN
3 doses/day
XARTEMIS XR
4 doses/day
DURAGESIC
1 patch/72 hr
NUCYNTA ER
2 doses/day
XTAMPZA ER
2 doses/day
EMBEDA
2 doses/day
OPANA ER
2 doses/day
ZOHYDRO ER
2 doses/day
EXALGO
1 dose/day
Approval Criteria
1. What is the patient’s diagnosis?
Record ICD10
2. Is the request for renewal of current therapy
previously approved by the FFS program?
Yes: Go to Renewal
Criteria
No: Go to #3
3. Is the requested medication a preferred
agent?
Yes: Go to #5
No: Go to #4
4. Will the prescriber change to a preferred
product?
Note:
Preferred opioids are reviewed and
designated as preferred agents by the Oregon
Pharmacy & Therapeutics Committee based
on published medical evidence for safety and
efficacy. Both oral and transdermal options
are available.
Yes: Inform prescriber of
covered alternatives in
class.
No: Go to #5
5. Is the patient being treated for cancer-related
pain (ICD10 G89.3) or under palliative care
services (ICD10 Z51.5) with a life-threatening
illness or severe advanced illness expected to
progress toward dying?
Yes: Approve for up to
12 months
No: Go to #6
6. Is the diagnosis funded by the OHP?
Yes: Go to #7
No: Pass to RPh. Go
to #15
7. Is the opioid prescription for pain associated
with a back or spine condition or for migraine
headache?
Yes: Pass to RPh. Go to
#15
No: Go to #8

Oregon Medicaid PA Criteria 152 July 1, 2017
8. Will the prescriber change to a preferred
product, not to exceed 90 MME per day and
not to exceed quantity limits in Table 2?
Note:
Preferred products that do not exceed 90
MME per day and do not exceed quantity
limits in Table 2 do not require prior
authorization.
Yes: Inform prescriber of
covered alternatives in
class.
No: Go to #9
9. Does the total daily opioid dose exceed 90
MME?
Yes: Pass to RPh. Go to
#15
No: Go to #10
10. Is the patient concurrently on other short- or
long-acting opioids (patients are permitted to
be on only one opioid product total at a time)?
Yes: Pass to RPh. Go to
#15
No: Go to #11
11. Does the prescription exceed quantity limits
applied in Table 2 (if applicable)?
Yes: Pass to RPh. Go to
#15
No: Go to #12
12. Can the prescriber provide documentation of
sustained improvement of both pain and
function in the past 3 months compared to
baseline (e.g., validated tools to assess
function include: Oswestry, Neck Disability
Index, SF-MPQ, and MSPQ)?
Yes: Go to #13
No: Pass to RPh. Go
to #15
13. Is the prescriber enrolled in the Oregon
Prescription Drug Monitoring Program
(PDMP) and has the prescriber verified at
least once in the past 3 months that the
patient has been prescribed analgesics by
only a single prescribing practice or prescriber
and has received those analgesics by only a
single pharmacy?
Yes: Go to #14
No: Pass to RPh. Go
to #15
14. Has the patient had a urinary drug screen
(UDS) within the past 1 year to verify absence
of illicit drugs and non-prescribed opioids?
Yes: Approve for up to 3
months. Subsequent
approvals will require:
Verification of
patient’s opioid claims
history in the Oregon
PDMP at least every
3 months
Documentation of
sustained
improvement in both
baseline pain and
function at least every
3 months
Documented UDS at
least every 12 months
No: Pass to RPh. Go
to #15

Oregon Medicaid PA Criteria 153 July 1, 2017
15. Is the request to initiate new opioid therapy or
to increase the total daily MME dose?
Yes: Pass to RPh. Deny;
medical appropriateness.
No: Pass to RPh.
Approve for 3 months.
Note:
Documentation of
progress towards
meeting all criteria in
this PA will be required
for approval of
subsequent claims.
All future opioid claims
are subject to
Renewal Criteria 3
months from this index
claim.
Renewal Criteria
1. Has the patient had a urinary drug screen
(UDS) within the past 1 year to verify absence
of illicit drugs and non-prescribed opioids?
Yes: Go to #2
No: Pass to RPh.
Deny; medical
appropriateness
2. Is the prescriber enrolled in the Oregon
Prescription Drug Monitoring Program
(PDMP) and has the prescriber verified at
least once in the past 3 months that the
patient has been prescribed analgesics by
only a single prescribing practice or prescriber
and has received those analgesics by only a
single pharmacy?
Yes: Go to #3
No: Pass to RPh.
Deny; medical
appropriateness
3. Can the prescriber provide documentation of
sustained improvement of both pain and
function in the past 3 months compared to
baseline (e.g., validated tools to assess
function include: Oswestry, Neck Disability
Index, SF-MPQ, and MSPQ)?
Yes: Go to #4
No: Pass to RPh.
Deny; medical
appropriateness
4. Does the prescription exceed quantity limits
applied in Table 2 (if applicable)?
Yes: Approve for up to 3
months if there is
documentation of an
individualized taper plan
with progress to meet the
quantity limits applied in
Table 2.
No: Go to #5 if not
applicable.
Without
documentation, pass
to RPh. Deny; medical
appropriateness.

Oregon Medicaid PA Criteria 154 July 1, 2017
Renewal Criteria
5. Is the patient concurrently on other short- or
long-acting opioids (patients are permitted to
be on only one opioid product total at a time)?
Yes: Approve for up to 3
months if there is
documentation of an
individualized taper plan
with progress to be
managed on one short-
or long-acting opioid only.
No: Go to #6 if not
applicable.
Without
documentation, pass
to RPh. Deny; medical
appropriateness.
6. Does the total daily opioid dose exceed 90
MME?
Yes: Approve for up to 3
months if there is
documentation of an
individualized taper plan
with progress toward
meeting ≤90 MME per
day.
No: Go to #7 if not
applicable.
Without
documentation, pass
to RPh. Deny; medical
appropriateness.
7. Is the diagnosis funded by the OHP?
Yes: Approve for up to 3
months. Subsequent
approvals will require:
Verification of
patient’s opioid claims
history in the Oregon
PDMP at least every
3 months
Documentation of
sustained
improvement in both
baseline pain and
function at least every
3 months
Documented UDS at
least every 12 months
No: Approve for up to
3 months if there is
documentation of an
individualized taper
plan with progress
toward tapering off
opioid.
Without
documentation, pass
to RPh. Deny; medical
appropriateness.
Clinical Notes:
How to Discontinue Opioids.
Adapted from the Washington State Interagency Guideline on Prescribing Opioids for Pain; Agency Medical Directors’ Group, June
2015. Available at http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf)
Selecting the optimal timing and approach to tapering depends on multiple factors. The rate of opioid taper should be
based primarily on safety considerations, and special attention is needed for patients on high dose opioids, as too rapid a
taper may precipitate withdrawal symptoms or drug-seeking behavior. In addition, behavioral issues or physical
withdrawal symptoms can be a major obstacle during an opioid taper. Patients who feel overwhelmed or desperate may
try to convince the provider to abandon the taper. Although there are no methods for preventing behavioral issues during
taper, strategies implemented at the beginning of chronic opioid therapy such as setting clear expectations and
development of an exit strategy are most likely to prevent later behavioral problems if a taper becomes necessary.
1. Consider sequential tapers for patients who are on chronic benzodiazepines and opioids. Coordinate care with other
prescribers (e.g. psychiatrist) as necessary. In general, taper off opioids first, then the benzodiazepines.
2. Do not use ultra-rapid detoxification or antagonist-induced withdrawal under heavy sedation or anesthesia (e.g.
naloxone or naltrexone with propofol, methohexital, ketamine or midazolam).
3. Establish the rate of taper based on safety considerations:

Oregon Medicaid PA Criteria 155 July 1, 2017
a. Immediate discontinuation if there is diversion or non-medical use,
b. Rapid taper (over a 2 to 3 week period) if the patient has had a severe adverse outcome such as overdose or
substance use disorder, or
c. Slow taper for patients with no acute safety concerns. Start with a taper of ≤10% of the original dose per week
and assess the patient’s functional and pain status at each visit.
4. Adjust the rate, intensity, and duration of the taper according to the patient’s response (e.g. emergence of opioid
withdrawal symptoms (see Table below)).
5. Watch for signs of unmasked mental health disorders (e.g. depression, PTSD, panic disorder) during taper, especially
in patients on prolonged or high dose opioids. Consult with specialists to facilitate a safe and effective taper. Use
validated tools to assess conditions.
6. Consider the following factors when making a decision to continue, pause or discontinue the taper plan:
a. Assess the patient behaviors that may be suggestive of a substance use disorder
b. Address increased pain with use of non-opioid options.
c. Evaluate patient for mental health disorders.
d. If the dose was tapered due to safety risk, once the dose has been lowered to an acceptable level of risk with
no addiction behavior(s) present, consider maintaining at the established lower dose if there is a clinically
meaningful improvement in function, reduced pain and no serious adverse outcomes.
7. Do not reverse the taper; it must be unidirectional. The rate may be slowed or paused while monitoring for and
managing withdrawal symptoms.
8. Increase the taper rate when opioid doses reach a low level (e.g. <15 mg/day MED), since formulations of opioids
may not be available to allow smaller decreases.
9. Use non-benzodiazepine adjunctive agents to treat opioid abstinence syndrome (withdrawal) if needed. Unlike
benzodiazepine withdrawal, opioid withdrawal symptoms are rarely medically serious, although they may be
extremely unpleasant. Symptoms of mild opioid withdrawal may persist for 6 months after opioids have been
discontinued (see Table below).
10. Refer to a crisis intervention system if a patient expresses serious suicidal ideation with plan or intent, or transfer to an
emergency room where the patient can be closely monitored.
11. Do not start or resume opioids or benzodiazepines once they have been discontinued, as they may trigger drug
cravings and a return to use.
12. Consider inpatient withdrawal management if the taper is poorly tolerated.
Symptoms and Treatment of Opioid Withdrawal.
Adapted from the Washington State Interagency Guideline on Prescribing Opioids for Pain; Agency Medical Directors’ Group, June
2015. Available at http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf)
Restlessness, sweating or
tremors
Clonidine 0.1-0.2 mg orally every 6 hours or transdermal patch 0.1-0.2 mg weekly (If using
the patch, oral medication may be needed for the first 72 hours) during taper. Monitor for
significant hypotension and anticholinergic side effects.
Nausea
Anti-emetics such as ondansetron or prochlorperazine
Vomiting
Loperamide or anti-spasmodics such as dicyclomine
Muscle pain, neuropathic
pain or myoclonus
NSAIDs, gabapentin or muscle relaxants such as cyclobenzaprine, tizanidine or
methocarbamol
Insomnia
Sedating antidepressants (e.g. nortriptyline 25 mg at bedtime or mirtazapine 15 mg at
bedtime or trazodone 50 mg at bedtime). Do not use benzodiazepines or sedative-
hypnotics.
P&T/DUR Review: 05/16 (AG)
Implementation: 1/1/17; 7/1/16

Oregon Medicaid PA Criteria 156 July 1, 2017
Oral Cystic Fibrosis Modulators
Goals:
To ensure appropriate drug use and limit to patient populations in which they have
demonstrated to be effective and safe.
To monitor for clinical response for appropriate continuation of therapy.
Length of Authorization:
90 days to 6 months
Requires PA:
Ivacaftor (Kalydeco
®
)
Lumacaftor/Ivacaftor (Orkambi
®
)
Preferred Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is this a request for continuation of therapy
previously approved by the FFS program
(patient already on ivacaftor or
lumacaftor/ivacaftor)?
Yes: Go to Renewal
Criteria
No: Go to #2
2. What diagnosis is being treated?
Record ICD10 code. Go to #3
3. Is the request from a practitioner at an
accredited Cystic Fibrosis Center or a
pulmonologist?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. How many exacerbations and/or
hospitalizations in the past 12 months has
the patient had?
Prescriber must provide documentation before
approval. Document baseline value.
Go to #5
5. Is the request for ivacaftor?
Yes: Go to #6
No: Go to #10
6. What is the patient’s baseline sweat chloride
level?
Prescriber must provide documentation before
approval. Document baseline value.
Go to #7
7. Does the patient have a diagnosis of cystic
fibrosis and is 2 years of age or older?
Yes: Go to #8
No: Pass to RPh. Deny;
medical appropriateness

Oregon Medicaid PA Criteria 157 July 1, 2017
Approval Criteria
8. Does the patient have a documented
G551D, G1244E, G1349D, G178R, G551S,
S1251N, S1255P, S549N, or S549R
mutation in the CFTR gene detected by an
FDA-cleared CF mutation test?
Yes: Go to #14
No: Go to #9
If unknown, there needs
to be a FDA-approved
CF mutation test to
detect the presence of
the CFTR mutation prior
to use.
CF due to other CFTR
gene mutations are not
approved indications
(including the F508del
mutation).
9. Does the patient have a documented R117H
mutation in the CFTR gene detected by an
FDA-cleared CF mutation test?
Yes: Pass to RPh.
Refer request to
Medical Director for
manual review and
assessment of clinical
severity of disease for
approval.
No: Pass to RPh. Deny;
medical appropriateness.
If unknown, there needs
to be a FDA-approved
CF mutation test to
detect the presence of
the CFTR mutation prior
to use.
CF due to other CFTR
gene mutations are not
approved indications
(including the F508del
mutation).
10. Is the request for lumacaftor/ivacaftor?
Yes: Go to #11
No: Pass to RPh. Deny;
medical appropriateness
11. Does the patient have a diagnosis of cystic
fibrosis and is 6 years of age or older?
Yes: Go to #12
No: Pass to RPh. Deny;
medical appropriateness

Oregon Medicaid PA Criteria 158 July 1, 2017
Approval Criteria
12. Does the patient have a documented
homozygous Phe508del mutation in the
CFTR gene detected by an FDA-approved
CF mutation test?
Yes: Go to #13
No: Pass to RPh. Deny;
medical appropriateness
If unknown, there needs
to be a FDA-approved
CF mutation test to
detect the presence of
the CFTR mutation prior
to use.
CF due to other CFTR
gene mutations are not
approved indications
(including those who are
heterozygous for the
F508del mutation)
13. Is a baseline FEV1 is provided and is
between ≥40% and ≤90% of predicted
normal for age, sex and height for those ≥12
years of age and at least 40% for children
ages 6 through 11 years?
Yes: If the patient is
younger than 12 years
of age, refer case to
OHP Medical Director;
otherwise, Go to #14
No: Pass to RPh. Deny;
medical appropriateness
If no baseline, request a
baseline value before
approving therapy.
14. Is the patient on ALL the following drugs, or
has had an adequate trial of each drug,
unless contraindicated or not appropriate
based on age <6 years and normal lung
function:
Dornase alfa; AND
Hypertonic saline; AND
Inhaled or oral antibiotics (if
appropriate)?
Yes: Go to #15
No: Pass to RPh. Deny;
medical appropriateness
15. Is the patient on concomitant therapy with a
strong CYP3A4 inducer (see Table 1)?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #16
16. What are the baseline liver function
(AST/ALT) and bilirubin levels (within
previous 3 months)?
Document labs. Go to #17

Oregon Medicaid PA Criteria 159 July 1, 2017
Approval Criteria
17. Is medication dosed appropriately based on
age, weight, and co-administered drugs (see
dosing and administration below)?
Yes: Approve for 90
days.
Note: Approve for 90
days to allow time for
patient to have a sweat
chloride test done after
30 days of treatment if
on ivacaftor (see
Renewal Criteria)
No: Pass to RPh. Deny;
medical appropriateness
Renewal Criteria
1. Is this the first time the patient is requesting
a renewal (after 90 days of initial approval)?
Yes: Go to #2
No: Go to #4
2. If prescription is for ivacaftor:
Does the patient have a documented
physiological response to therapy and
evidence of adherence after 30 days of
treatment, as defined by a sweat chloride
test that has decreased by at least 20
mmol/L from baseline?
Yes: Go to #7
No: Go to #3
Consider patient’s
adherence to therapy
and repeat test in 2
weeks to 45 days to
allow for variability in
test. If sodium chloride
has still not decreased
by 20 mmol/L, deny
therapy for medical
appropriateness
3. If the prescription is for lumacaftor/ivacaftor:
Is there evidence of adherence and
tolerance to therapy through pharmacy
claims/refill history and provider
assessment?
Yes: Go to #7
No: Pass to RPh; Deny
(medical
appropriateness)

Oregon Medicaid PA Criteria 160 July 1, 2017
Renewal Criteria
4. Does the patient have documented
response to therapy as defined as below :
For patients age ≥6 years:
An improvement or lack of decline in
lung function as measured by the
FEV1 when the patient is clinically
stable; OR
A reduction in the incidence of
pulmonary exacerbations; OR
A significant improvement in BMI by
10% from baseline?
For patients age 2-5 years (cannot complete
lung function tests)
Significant improvement in BMI by
10% from baseline; OR
Improvement in exacerbation
frequency or severity; OR
Sweat chloride test has decreased
from baseline by 20 mmol/L from
baseline?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness
5. Has the patient been compliant with therapy,
as determined by refill claims history?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness
6. Have liver function tests been appropriately
monitored? What are the most recent liver
function tests (AST, ALT, and bilirubin)?
Note: Monitoring LFTs is recommended
every 3 months for the first year, followed by
once a year.
Document. Go to #7
Note: Therapy should be interrupted in patients
with AST or ALT >5x the upper limit of normal
(ULN), or ALT or AST >3x ULN with bilirubin >2x
ULN.
7. Is the CFTR modulator dosed appropriately
based on age, weight, and co-administered
drugs (see dosing and administration
below)?
Yes: Approve for
additional 3 months
(total of 6 months since
start of therapy)
No: Pass to RPh. Deny;
medical
appropriateness
Dosage and Administration:
Ivacaftor:
Adults and pediatrics age ≥6 years: 150 mg orally every 12 hours with fat-containing foods
Children age 2 to <6 years:

Oregon Medicaid PA Criteria 161 July 1, 2017
o < 14 kg: 50 mg packet every 12 hours
o ≥ 14 kg: 75 mg packet every 12 hours
Hepatic Impairment
o Moderate Impairment (Child-Pugh class B):
Age ≥6 years: one 150 mg tablet once daily
Age 2 to < 6 years with body weight < 14 kg: 50 mg packet once daily; with body
weight ≥ 14 kg : 75 mg packet of granules once daily
o Severe impairment (Child-Pugh class C): Use with caution at a dose of 1 tablet or 1
packet of oral granules once daily or less frequently.
Dose adjustment with concomitant medications:
Table 1. Examples of CYP3A4 inhibitors and inducers.
Drug co-administered
with ivacaftor
Co-administered
drug category
Recommended dosage adjustment for ivacaftor
Ketoconazole
Itraconazole
Posaconazole
Voriconazole
Clarithromycin
Telithromycin
CYP3A4 strong
inhibitors
Reduce ivacaftor dose to 1 tablet or 1 packet of oral
granules twice weekly (one-seventh of normal initial
dose)
Fluconazole
Erythromycin
Clofazimine
CYP3A4 moderate
inhibitors
Reduce ivacaftor dose to 1 tablet or 1 packet of oral
granules once daily (half of normal dose)
Rifampin
Rifabutin
Phenobarbital
Phenytoin
Carbamazepine
St. John’s wort
Grapefruit Juice
CYP3A4 strong
inducers
Concurrent use is NOT recommended
Lumacaftor/ivacaftor:
Adults and pediatrics age ≥12 years: 2 tablets (lumacaftor 200 mg/ivacaftor 125 mg) every 12
hours
Pediatric patients age 6 through 11 years: 2 tablets (lumacaftor 100mg/ivacaftor 125 mg) every
12 hours
Hepatic impairment
o Moderate impairment (Child-Pugh class B):
2 tablets in the morning and 1 tablet in the evening
o Severe impairment (Child-Pugh class C): Use with caution at a dose of 1 tablet twice
daily, or less, after weighing the risks and benefits of treatment.
Dose adjustment with concomitant medications:
o When initiating therapy in patients taking strong CYP3A inhibitors (see table above),
reduce dose to 1 tablet daily for the first week of treatment. Following this period,
continue with the recommended daily dose.
P&T Review: 11/16 (MH); 11/15; 7/15; 5/15; 5/14; 6/12
Implementation: TBD; 1/1/16; 8/25/15; 8/12

Oregon Medicaid PA Criteria 162 July 1, 2017
Oxazolidinone Antibiotics
Goal(s):
To optimize treatment of infections due to gram-positive organisms such as methicillin-resistant
Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE)
Length of Authorization:
6 days
Requires PA:
Non-preferred Oxazolidinone antibiotics
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD-10 code.
2. Does the patient have an active infection
with suspected or documented MRSA (e.g.
B95.8, B95.61, B95.62, J15212) or VRE
(e.g. Z16.20, Z16.21, Z16.22, Z16.31,
Z16.32, Z16.33, Z16.39) or other multi-drug
resistant gram-positive cocci (e.g. Z16.30,
Z16.24)?
Yes: Go to #3.
No: Pass to RPh. Deny;
medical appropriateness
3. Does the patient have a documented trial of
appropriate therapy with vancomycin or
linezolid, or is the organism not susceptible?
Yes: Approve
tedizolid for up to 6
days and other non-
preferred drugs for
prescribed course.
No: Pass to RPh. Deny;
medical appropriateness
P&T/DUR Review: 5/15
Implementation 10/13/16; 7/1/15

Oregon Medicaid PA Criteria 163 July 1, 2017
Palivizumab (Synagis
®
)
Goal(s):
Promote safe and effective use of palivizumab.
Length of Authorization:
Based on individual factors; may extend up to 5 months (5 doses)
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Has the patient been receiving monthly
palivizumab prophylaxis and been
hospitalized for a breakthrough RSV
infection?
Yes: Pass to RPh; deny for
medical appropriateness.
No: Go to #3
3. Is the request for immunoprophylaxis
between the months of November and
March?
Yes: Go to #5
No: Go to #4
4. Is the request for immunoprophylaxis
starting in October due to an early onset*
of the RSV season in the region from
which the patient resides (see below)?
* Onset is defined as 2 consecutive weeks where % positive is
≥10%, (data are provided by the Oregon’s Weekly Respiratory
Syncytial Virus Surveillance Report from the Oregon Public Health
Division based on regions. Weekly updates are found at:
https://public.health.oregon.gov/DiseasesConditions/DiseasesAZ/P
ages/disease.aspx?did=40)
Region
Counties
NW Oregon –
SW Washington
Benton, Clackamas, Clatsop,
Columbia, Lane, Lincoln, Linn,
Marion, Multnomah, Polk,
Tillamook, Washington, Yamhill
Central Oregon
Crook, Deschutes, Grant,
Harney, Jefferson, Wheeler
Columbia Gorge
– NE Oregon
Baker, Gilliam, Hood River,
Morrow, Sherman, Umatilla,
Union, Wasco, Wallowa
Southern
Oregon
Coos, Curry, Douglas, Jackson,
Josephine, Klamath, Lake,
Malheur
Yes: Go to #5
No: Pass to RPh.
Deny; medical
appropriateness.
Prophylaxis is
indicated only during
high viral activity.
5. Is the current age of the patient < 24
months at start of RSV season?
Yes: Go to #6
No: Pass to RPh.
Deny; medical
appropriateness. Not
recommended for
patients ≥24 months
old.

Oregon Medicaid PA Criteria 164 July 1, 2017
Approval Criteria
6. GROUP A
Does the patient have the CLD (chronic
lung disease) of prematurity ICD10
Q331through Q339 and in the past 6
months has required medical treatment
with at least one of the following:
a. diuretics
b. chronic corticosteroid therapy
c. supplemental oxygen therapy
Yes: Go to #18
No: Go to #7
7. GROUP B
Has the patient received a cardiac
transplant during the RSV season?
Yes: Go to #18
No: Go to #8
8. GROUP C
Is the child profoundly
immunocompromised during the RSV
season (i.e. solid organ transplant or
hematopoietic stem cell transplantation)?
Yes: Go to #18
No: Go to #9
9. GROUP D
Does the infant have cystic fibrosis and
manifestations of severe lung disease or
weight or length less than the 10
th
percentile?
Yes: Go to #18
No: Go to #10
10. GROUP E
Is the request for a second season of
palivizumab prophylaxis for a child born
<32 weeks, 0 days gestation who
required at least 28 days of oxygen,
chronic systemic corticosteroid therapy,
or bronchodilator therapy within 6
months of start of second RSV season?
Yes: Go to #18
No: Go to #11
11. Will the patient be <12 months at start of
RSV season?
Yes: Go to #12
No: Pass to RPh.
Deny; medical
appropriateness.
12. GROUP F
Was the infant born before 29 weeks, 0
days gestation?
Yes: Go to #18
No: Go to #13

Oregon Medicaid PA Criteria 165 July 1, 2017
Approval Criteria
13. GROUP G
Does the infant have pulmonary
abnormalities of the airway or
neuromuscular disease compromising
handling of secretions?
Yes: Go to #18
No: Go to #14
14. GROUP H
Does the patient have hemodynamically
significant congenital heart disease
(CHD) ICD10: P293, Q209, Q220-Q223,
Q225, Q229-Q234, Q238, Q240-Q246,
Q248-Q249, Q250-Q256, Q278-
Q279,Q282-Q283,Q288-Q289, Q2560-
Q2565,Q2568-Q2569, Q2570-Q2572,
Q2579,Q2731-Q2732 and at least one of
the following:
a. Acyanotic heart disease who are
receiving treatment to control congestive
heart failure and will require cardiac
surgical procedures; OR
b. Have moderate to severe pulmonary
hypertension; OR
c. History of lesions adequately
corrected by surgery AND still requiring
medication for congestive heart failure?
Yes: Go to #18
No: Go to #15
15. GROUP I
Does the patient have chronic lung
disease (CLD) of prematurity defined as
gestational age <32 weeks, 0 days and
requirement for >21% oxygen for at least
the first 28 days after birth?
Yes: Go to #18
No: Go to #16
16. GROUP J
Does the patient have cyanotic heart
defects and immunoprophylaxis is
recommended?
Yes: Go to #18
No: Go to #17
17. GROUP K
Does the patient have cystic fibrosis with
clinical evidence of CLD and/or
nutritional compromise?
Yes: Go to #18
No: Pass to RPh.
Deny; medical
appropriateness.
Oregon Medicaid PA Criteria 166 July 1, 2017
Approval Criteria
18. Is the request for more than 5 doses
within the same RSV season or for
dosing <28 days apart?
Yes: Pass to RPh. Deny;
medical appropriateness.
Prophylaxis is indicated for 5
months maximum and doses
should be administered >28
days apart.
May approve for the following
on a case-by-case basis:
a. >5 doses;
b. Prophylaxis for a second /
subsequent RSV season
No: Go to #19
19. Has the patient had a weight taken within
the last 30 days?
Yes: Document weight and
date and go to #20
Weight:_______
Date:_________
No: Pass to RPh.
Obtain recent weight
so accurate dose can
be calculated.
20. Approve palivizumab for a dose of 15 mg/kg. Document number of doses received in hospital and
total number approved according to BIRTH DATE and GROUP based on start of RSV season:
- Immunoprophylaxis between November - March refer to Table 1
- Immunoprophylaxis starting in October based on above (#4) refer to Table 2
Total number of doses approved for RSV season:________
Number of doses received in the hospital:________
Prior to each refill, the patient’s parent/caregiver and prescriber must comply with all case
management services, including obtaining current weight for accurate dosing purposes throughout
the approved treatment period as required by the Oregon Health Authority.
Table 1. Maximum Number of Doses for RSV Prophylaxis (based on criteria group from above)
Beginning NOVEMBER 1
MONTH OF BIRTH
ALL GROUPS
November 1 – March 31
5
April
5
May
5
June
5
July
5
August
5
September
5
October
5
November
5
December
4
January
3
February
2
March
1

Oregon Medicaid PA Criteria 167 July 1, 2017
* Infant may require less doses than listed based on age at the time of discharge from the hospital. Subtract number of doses given
in hospital from total number of approved doses.
Table 2. Maximum Number of Doses for RSV Prophylaxis (based on criteria group from above)
Beginning OCTOBER 1
MONTH OF BIRTH
ALL GROUPS
November 1 – March 31
5
April
5
May
5
June
5
July
5
August
5
September
5
October
5
November
5
December
4
January
3
February
2
March
1
* Infant may require less doses than listed based on age at the time of discharge from the hospital. Subtract number of doses given
in hospital from total number of approved doses.
Notes:
- Dose: 15 mg/kg via intramuscular injection once monthly throughout RSV season.
- The start date for Synagis
®
is November 1 each year (or sooner when the Oregon Public Health Division has
determined that RSV season onset has occurred) for a total of up to 5 doses.
- Approval for more than 5 doses or additional doses after March 31 will be considered on a case-by-case basis.
Results from clinical trials indicate that Synagis
®
trough concentrations greater than 30 days after the 5
th
dose are
well above the protective concentration. Therefore, 5 doses will provide more than 20 weeks of protection.
P&T/DUR Review: 11/16 (DE); 9/14; 5/11; 5/12
Implementation: 1/1/17; 3/30/12

Oregon Medicaid PA Criteria 168 July 1, 2017
Patiromer
Goals:
Restrict use of patiromer to patients with persistent or recurrent hyperkalemia not requiring
urgent treatment.
Prevent use in the emergent setting or in scenarios not supported by the medical literature.
Encourage use to optimize medications with demonstrated evidence of mortality reduction in
heart failure with reduced ejection fraction.
Length of Authorization:
6 to 12 months
Requires PA:
Patiromer
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is this a request for continuation of therapy
previously approved by the FFS program
(patient already on patiromer)?
Yes: Go to Renewal
Criteria
No: Go to #2
2. What diagnosis is being treated?
Record ICD10 code. Go to #3
3. Does the patient have persistent or
recurrent serum potassium of ≥5.5 mEq/L
despite a review for discontinuation of
medications that may contribute to
hyperkalemia (e.g., potassium supplements,
potassium-sparing diuretics, nonsteroidal
anti-inflammatory drugs)?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Has the patient tried and failed or cannot
tolerate sodium polystyrene?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness
5. Does the patient have hyperkalemia
requiring emergency intervention (serum
potassium ≥6.5 mEq/L)?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #6
6. Does the patient have hypomagnesemia
(serum magnesium < 1.4 mg/dL)?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #7
Oregon Medicaid PA Criteria 169 July 1, 2017
Approval Criteria
7. Does the patient have a severe GI disorder
(i.e., major GI surgery (e.g., large bowel
resection), bowel obstruction/impaction,
swallowing disorders, gastroparesis, severe
constipation)?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Approve up to 6
months
Renewal Criteria
1. Is the patient’s potassium level < 5.1 mEq/L
and has this decreased by at least 0.35
mEq/L from baseline?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
medical
appropriateness
P&T Review: 05/16 (EL/MH)
Implementation: 8/16, 7/1/16
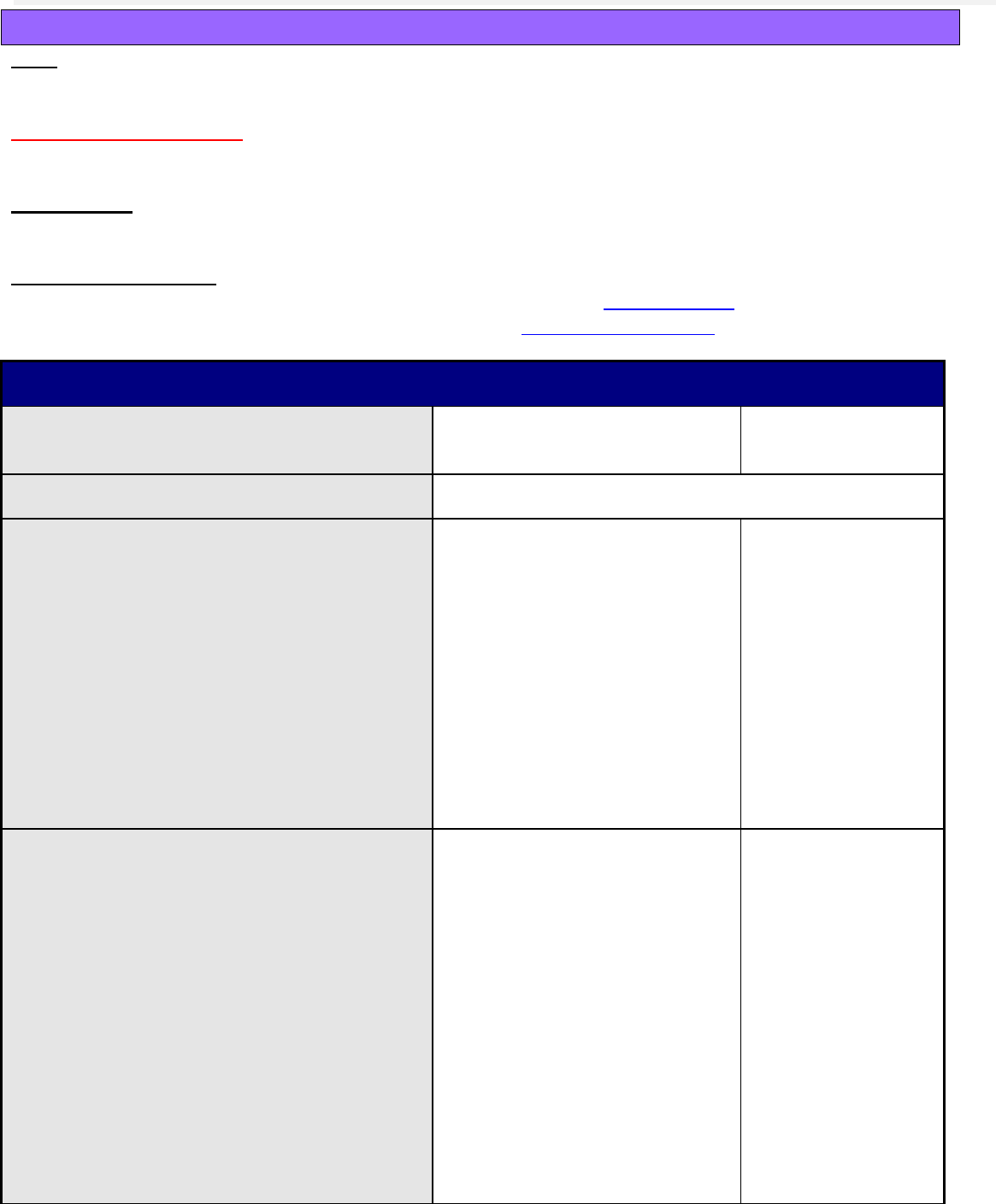
Oregon Medicaid PA Criteria 170 July 1, 2017
PCSK9 Inhibitors
Goal:
Restrict use of PCSK9 inhibitors to populations in which the drugs have demonstrated efficacy.
Length of Authorization:
Up to 12 months
Requires PA:
All PCSK9 inhibitors
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is this a request for renewal of a
previously approved prior authorization?
Yes: Go to Renewal Criteria
No: Go to #2
2. What diagnosis is being treated?
Record ICD10 code. Go to #3
3. Does the patient have clinical
atherosclerotic CV disease, defined as
documented history of ≥1 of the following:
Myocardial infarction; OR
Unstable angina; OR
Coronary revascularization procedure
(PCI or CABG); OR
Diagnosis of clinically significant
coronary heart disease by coronary
angiography, stress test using
treadmill, stress echocardiography or
nuclear imaging?
Yes: Go to #4
No: Go to #6
4. Has the patient taken a daily high-
intensity statin (see table below) and
ezetimibe 10 mg daily for at least 12
months with <50% LDL-C reduction?
Prescriber to submit chart documentation
of:
1) Doses and dates initiated of statin and
ezetimibe;
2) Baseline LDL-C (untreated);
3) Recent LDL-C (within last 12 weeks).
Yes: Confirm documentation;
go to #5
1. Statin:
Dose:
Date Initiated:
2. Ezetimibe 10 mg daily
Date Initiated:
Baseline LDL-C ______ mg/dL
Date:_________
Recent LDL-C ______ mg/dL
Date:_________
No: Go to #6
Oregon Medicaid PA Criteria 171 July 1, 2017
Approval Criteria
5. Is the patient adherent with a high-
intensity statin and ezetimibe?
Yes: Approve for up to 12
months
Note: pharmacy profile may be
reviewed to verify >80%
adherence (both lipid-lowering
prescriptions refilled 5 months’
supply in last 6 months)
No: Pass to RPh.
Deny; medical
appropriateness
6. Does the patient have a history of
rhabdomyolysis caused by a statin; or
alternatively, a history of creatinine
kinase (CK) levels >10-times upper limit
of normal with muscle symptoms
determined to be caused by a statin?
Note: Prescriber must provide chart
documentation of diagnosis or CK levels.
A recent LDL-C level (within last 12
weeks) must also be submitted.
Yes: Confirm chart
documentation of diagnosis or
labs and approve for up to 12
months
Recent LDL-C ______ mg/dL
Date:_________
No: Go to #7
7. Does the patient have a diagnosis of
homozygous or heterozygous familial
hypercholesterolemia and already takes a
maximally tolerated statin and/or
ezetimibe?
Note: Prescriber must provide chart
documentation of diagnosis and recent
LDL-C (within last 12 weeks).
Yes: Document diagnosis and
approve for up to 12 months
Recent LDL-C ______ mg/dL
Date:_________
No: Pass to RPh.
Deny; medical
appropriateness.
Renewal Criteria
1. What is the most recent LDL-C (within
last 12 weeks)?
Recent LDL-C ______ mg/dL
Date:_________ . Go to #2
2. Is the patient adherent with PCSK9
inhibitor therapy?
Yes: Approve for up to 12
months
Note: pharmacy profile may be
reviewed to verify >80%
adherence (PCSK9 inhibitor
prescription refilled 10 months’
supply in last 12 months)
No: Pass to RPh.
Deny; medical
appropriateness
High- and Moderate-intensity Statins. Stone NJ, et al. 2013 ACC/AHA Blood Cholesterol Guideline.

Oregon Medicaid PA Criteria 172 July 1, 2017
High-intensity Statins
(50% LDL-C Reduction)
Moderate-intensity Statins
(30 to <50% LDL-C Reduction)
Atorvastatin 40-80 mg
Rosuvastatin 20-40 mg
Atorvastatin 10-20 mg
Fluvastatin 80 mg
Lovastatin 40 mg
Pitavastatin 2-4 mg
Pravastatin 40-80 mg
Simvastatin 20-40 mg
Rosuvastatin 5-10 mg
References:
1. NICE Clinical Guideline 181. Lipid modification: CV risk assessment and the modification of blood lipids for the primary and
secondary prevention of CV disease. Available at: guidance.nice.org.uk/cg181. Accessed 18 September 2015.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce
Atherosclerotic CV Risk in Adults. A report of the American College of Cardiology/American Heart Association Task Force on Practice
Guidelines. Circulation. 2013;129(25 Suppl 2):S1-45. doi: 10.1161/01.cir.0000437738.63853.7a.
P&T/DUR Review: 11/16 (DM); 11/15
Implementation: 1/1/17

Oregon Medicaid PA Criteria 173 July 1, 2017
Preferred Drug List (PDL) – Non-Preferred Drugs in Select PDL Classes
Goal(s):
Ensure that non-preferred drugs are used appropriately for OHP-funded conditions.
Initiative:
PDL: Preferred Drug List
Length of Authorization:
Up to 6 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is this an FDA approved indication?
Yes: Go to #3
No: Pass to RPh. Deny;
medical
appropriateness
3. Is this an OHP-funded diagnosis?
Yes: Go to #4
No: Go to #5
4. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not generally require
a PA.
Preferred products are evidence-based and
reviewed for comparative effectiveness and
safety by the P&T Committee.
Yes: Inform prescriber of
covered alternatives in
class.
No: Approve until
anticipated formal
review by the P&T
committee, for 6
months, or for length of
the prescription,
whichever is less.
5. RPh only: All other indications need to be evaluated as to whether they are a funded diagnosis on
the OHP prioritized list.
If funded and clinic provides supporting literature: Approve until anticipated formal review by
the P&T committee, for 6 months, or for length of the prescription, whichever is less.
If not funded: Deny; not funded by the OHP.
P&T / DUR Review: 7/15 (RC), 9/10; 9/09; 5/09
Implementation: 10/13/16; 8/25/15; 8/15; 1/1/11, 9/16/10

Oregon Medicaid PA Criteria 174 July 1, 2017
Peginterferon Beta-1a (Plegridy®)
Goal(s):
Approve therapy for covered diagnosis which are supported by the medical literature.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Does the patient have a diagnosis of
relapsing-remitting Multiple Sclerosis?
Yes: Go to #3.
No: Pass to RPH; Deny
for medical
appropriateness.
3. Will the prescriber consider a change to a
Preferred MS product?
Yes: Inform provider of
covered alternatives in
the class. Additional
information can be found
at www.orpdl.org.
No: Go to #4.
4. Is the medication being prescribed by or in
consultation with a neurologist?
Yes: Go to #5.
No: Pass to RPH; Deny
for medical
appropriateness.
5. Does the patient have any of the following:
Adherence issues necessitating less
frequent administration
Dexterity issues limiting ability to
administer subcutaneous injections
Yes: Approve for up to
one year.
No: Pass to RPH; Deny
for medical
appropriateness.
P&T / DUR Action: 9/23/14 (KS)
Implementation: 10/15

Oregon Medicaid PA Criteria 175 July 1, 2017
Pegylated Interferons and Ribavirins
Goal(s):
Cover drugs only for those clients where there is medical evidence of effectiveness and safety
Length of Authorization:
16 weeks plus 12-36 additional weeks or 12 months
Requires PA:
All drugs in HIC3 = W5G
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is peginterferon requested preferred?
Yes: Go to #4
No: Go to #2
2. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products are evidence-based
reviewed for comparative effectiveness &
safety Oregon Pharmacy and Therapeutics
(P&T) Committee
Yes: Inform provider of
covered alternatives in
class.
No: Go to #3
3. If the request is for interferon alfacon-1,
does the patient have a documented trial of
a pegylated interferon?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Is the request for treatment of Chronic
Hepatitis C?
Document appropriate ICD10 code:
(K739; K730; K732 or K738)
Yes: Go to #5
No: Go to #11
5. Is the request for continuation of therapy
previously approved by the FFS program?
(Patient has been on HCV treatment in the
preceding 12 weeks according to the Rx
profile)
Yes: Go to
“Continuation of
Therapy”
No: Go to #6
Oregon Medicaid PA Criteria 176 July 1, 2017
Approval Criteria
6. Does the patient have a history of treatment
with previous pegylated interferon-ribavirin
combination treatment?
Verify by reviewing member’s Rx profile for
PEG-Intron or Pegasys, PLUS ribavirin
history. Does not include prior treatment
with interferon monotherapy or non-
pegylated interferon.
Yes: Forward to DMAP
Medical Director
No: Go to #7
7. Does the patient have any of the following
contraindications to the use of interferon-
ribavirin therapy?
severe or uncontrolled psychiatric
disorder
decompensated cirrhosis or hepatic
encephalopathy
hemoglobinopathy
untreated hyperthyroidism
severe renal impairment or transplant
autoimmune disease
pregnancy
unstable CVD
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #8
8. If applicable, has the patient been abstinent
from IV drug use or alcohol abuse for ≥ 6
months?
Yes: Go to #9
No: Pass to RPh. Deny;
medical
appropriateness
9. Does the patient have a detectable HCV
RNA (viral load) > 50IU/mL? Record HCV
RNA and date.
Yes: Go to #10
No: Pass to RPh. Deny;
medical
appropriateness

Oregon Medicaid PA Criteria 177 July 1, 2017
Approval Criteria
10. Does the patient have a documented HCV
Genotype? Record Genotype.
Yes: Approve for 16
weeks with the following
response: Your request
for has been approved
for an initial 16 weeks.
Subsequent approval is
dependent on
documentation of
response via a repeat
viral load demonstrating
undetectable or 2-log
reduction in HCV viral
load. Please order a
repeat viral load after 12
weeks submit lab results
and relevant medical
records with a new PA
request for continuation
therapy.
Note: For ribavirin
approve the generic
only.
No: Pass to RPh. Deny;
medical
appropriateness
11. Is the request for Pegasys and the
treatment for confirmed, compensated
Chronic Hepatitis B?
Yes: Go to #11
No: Pass to RPh. Deny;
medical
appropriateness
12. Is the patient currently on LAMIVUDINE
(EPIVIR HBV), ADEFOVIR (HEPSERA),
ENTECAVIR (BARACLUDE),
TELBIVUDINE (TYZEKA) and the request is
for combination Pegasys-oral agent
therapy?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #12
13. Has the member received previous
treatment with pegylated interferon?
Yes: Pass to RPh.
Deny; medical
appropriateness
Recommend:
LAMIVUDINE (EPIVIR
HBV) ADEFOVIR
(HEPSERA)
No: Approve
Pegasys #4 x 1mL vials
or #4 x 0.5 mL syringes
per month for 12
months (maximum per
lifetime).

Oregon Medicaid PA Criteria 178 July 1, 2017
Continuation of Therapy- HCV
1. Does the client
have undetectable
HCV RNA or at
least a 2-log
reduction (+/- one
standard
deviation) in HCV
RNA measured at
12 weeks?
Yes: Approve as follows:
Approval for beyond quantity and duration limits
requires approval from the medical director.
Geno-
type
Approve for:
Apply
1 or 4
An additional 36
weeks or for up to
a total of 48 weeks
of therapy
(whichever is the
lesser of the two).
Ribavirin quantity
limit of 200 mg
tablets QS# 180 /
25 days (for max
daily dose =1200
mg).
2 or 3
An additional 12
weeks or for up to
a total of 24 weeks
of therapy
(whichever is the
lesser of the two).
Ribavirin quantity
limit of 200 mg tab
QS# 120 / 25 days
(for max daily dose
= 800 mg).
For all
genotyp
es and
HIV co-
infection
An additional 36
weeks or for up to
a total of 48 weeks
of therapy
(whichever is the
lesser of the two)
Ribavirin quantity
limit of 200 mg
tablets QS# 180 /
25 days (for max
daily dose = 1200
mg).
No: Pass to RPh. Deny;
medical appropriateness
Treatment with
pegylated interferon-
ribarvirin does not meet
medical necessity
criteria because there is
poor chance of
achieving an SVR.
Clinical Notes:
• Serum transaminases: Up to 40% of clients with chronic hepatitis C have normal serum
alanine aminotransferase (ALT) levels, even when tested on multiple occasions.
• RNA: Most clients with chronic hepatitis C have levels of HCV RNA (viral load) between
100,000 (105) and 10,000,000 (107) copies per ml. Expressed as IU, these averages are 50,000 to 5
million IU. Rates of response to a course of peginterferon-ribavirin are higher in clients with low levels
of HCV RNA. There are several definitions of a “low level” of HCV RNA, but the usual definition is
below 800,000 IU (~ 2 million copies) per ml (5).
• Liver biopsy: Not necessary for diagnosis but helpful for grading the severity of disease and
staging the degree of fibrosis and permanent architectural damage and for ruling out other causes of
liver disease, such as alcoholic liver injury, nonalcoholic fatty liver disease, or iron overload.

Oregon Medicaid PA Criteria 179 July 1, 2017
Stage is indicative of fibrosis:
Grade is indicative of necrosis:
Stage 0
No fibrosis
Stage 1
Enlargement of the portal areas by
fibrosis
Stage 1
None
Stage 2
Fibrosis extending out from the portal
areas with rare bridges between portal
areas
Stage 2
Mild
Stage 3
Fibrosis that link up portal and central
areas of the liver
Stage 3
Moderate
Stage 4
Cirrhosis
Stage 4
Marked
The following are considered investigational and/or do not meet medical necessity criteria:
Treatment of HBV or HCV in clinically decompensated cirrhosis
Treatment of HCV or HBV in liver transplant recipients
Treatment of HCV or HBV > 48 weeks
Treatment of advanced renal cell carcinoma
Treatment of thrombocytopenia
Treatment of human papilloma virus
Treatment of multiple myeloma
P&T Review: 2/12; 9/09; 9/05; 11/04; 5/04
Implementation: 8/16, 5/14/12, 1/1/10, 5/22/08

Oregon Medicaid PA Criteria 180 July 1, 2017
Phosphate Binders
Goal(s):
Promote use of preferred drugs.
Reserve non-calcium-based phosphate binders for second-line therapy.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred phosphate binders
Preferred non-calcium-based phosphate binders
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is this an OHP-funded diagnosis?
Yes: Go to #3
No: Go to #5
3. Has the patient tried or contraindicated to
calcium acetate?
Yes: Document trial
dates and/or intolerance.
Go to #4
No: Pass to RPh. Deny;
medical
appropriateness.
Recommend trial of
preferred calcium
acetate product.
4. Will the prescriber consider a change to a
preferred non-calcium-based phosphate
binder?
Yes: Approve for 1 year
and inform prescriber of
preferred alternatives in
class.
No: Approve for 1 year
or length of prescription,
whichever is less.
5. RPh only: All other indications need to be evaluated as to whether use is for an OHP-funded
diagnosis.
If funded and clinic provides supporting literature, approve for up to 12 months.
If non-funded, deny; not funded by the OHP.
P&T Review: 1/16 (AG); 11/12; 9/12; 9/10
Implementation: 5/1/16; 2/21/13

Oregon Medicaid PA Criteria 181 July 1, 2017
Pimavanserin (Nuplazid™) Safety Edit
Goals:
Promote safe use of pimavanserin in patients with psychosis associated with Parkinson’s disease.
Length of Authorization:
Up to 6 months
Requires PA:
Pimavanserin
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the treatment for hallucinations and/or
delusions associated with Parkinson’s
disease?
Yes: Go to #3
No: Pass to RPh. Deny;
medical
appropriateness
3. Are the symptoms likely related to a change
in the patient’s anti-Parkinson’s medication
regimen?
Yes: Go to #4
Consider slowly
withdrawing medication
which may have
triggered psychosis.
No: Go to #5
4. Has withdrawal or reduction of the triggering
medication resolved symptoms?
Yes: Pass to RPh;
Deny; medical
appropriateness
No: Go to #5
5. Is the patient on a concomitant first- or
second-generation antipsychotic drug?
Yes: Pass to RPh;
Deny; medical
appropriateness
No: Go to #6
6. Has the patient been recently evaluated for
a prolonged QTc interval?
Yes: Approve for up to 6
months
No: Pass to RPh; Deny;
medical
appropriateness
P&T Review: 01/2017 (SS)
Implementation: 4/1/17

Oregon Medicaid PA Criteria 182 July 1, 2017
Pregabalin
Goal(s):
Provide coverage only for funded diagnoses that are supported by the medical literature.
Length of Authorization:
90 days to lifetime (criteria-specific)
Requires PA:
Pregabalin
Covered Alternatives
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is this a request for renewal of a previously
approved prior authorization for pregabalin?
Yes: Go to
Renewal
Criteria
No: Go to # 2
2. What diagnosis is being treated?
Record ICD10 code
3. Does the patient have a diagnosis of epilepsy?
Yes: Approve
for lifetime
No: Go to # 4
4. Is the diagnosis an OHP-funded diagnosis with
evidence supporting its use in that condition
(see Table 1 below for examples)?
Yes: Go to # 5
No: Pass to RPh. Deny; not
funded by the OHP.
5. Has the patient tried and failed gabapentin
therapy for 90 days or have contradictions or
intolerance to gabapentin?
Yes: Approve
for 90 days
No: Pass to RPh. Deny and
recommend trial of
gabapentin for 90 days
Renewal Criteria
1. Does the patient have documented
improvement from pregabalin?
Yes: Approve
for up to 12
months
No: Pass to RPh. Deny for
medical appropriateness

Oregon Medicaid PA Criteria 183 July 1, 2017
Table 1. OHP Funded Diagnosis and Evidence Supports Drug Use in Specific Indication
Condition
Pregabalin
Funded
Diabetic Neuropathy
X
Postherpetic
Neuropathy
X
Painful
Polyneuropathy
X
Spinal Cord Injury
Pain
X
Chemotherapy
Induced Neuropathy
X
Non-funded
Fibromyalgia
X
P&T Review: 3/17 (DM)
Implementation: 4/1/17

Oregon Medicaid PA Criteria 184 July 1, 2017
Proton Pump Inhibitors (PPIs)
Goals:
Promote PDL options
Restrict PPI use to patients with OHP-funded conditions
Requires PA:
Preferred PPIs beyond 68 days’ duration
Non-preferred PPIs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Individual components for treatment of H. pylori that are preferred products
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the request for a preferred PPI?
Yes: Go to #5
No: Go to #3
3. Is the treating diagnosis an OHP-funded
condition (see Table)?
Yes: Go to #4
No: Pass to RPh; deny,
not funded by OHP.
4. Will the prescriber consider changing to a
preferred PPI product?
Message: Preferred products are reviewed for
comparative effectiveness and safety by the
Pharmacy and Therapeutics (P&T) Committee.
Yes: Inform
prescriber of
covered
alternatives.
No: Go to #5
5. Has the patient already received 68 days of PPI
therapy for either of the following diagnoses:
Esophagitis or gastro-esophageal reflux
disease with or without esophagitis (K20.0-
K21.9); or
Current H. pylori infection?
Yes: Go to #6
No: Go to #7

Oregon Medicaid PA Criteria 185 July 1, 2017
6. Does the patient have recurrent, symptomatic
erosive esophagitis that has resulted in
previous emergency department visits or
hospitalizations?
Yes: Approve for 1
year
No: Go to #7
7. Does the patient have a history of
gastrointestinal ulcer or bleed and have one or
more of the following risk factors?
a. Age 65 years or older
b. Requires at least 3 months of continuous
daily:
i. Anticoagulant;
ii. Aspirin or non-selective NSAID; or
iii. Oral corticosteroid
Yes: Approve for 1
year
No: Go to #8
8. Are the indication, daily dose and duration of
therapy consistent with criteria outlined in the
Table?
Message: OHP-funded conditions are listed in the
Table.
Yes: Approve for
recommended
duration.
No: Pass to RPh. Deny;
medical appropriateness
or not funded by OHP
Message: Patient may
only receive 8 weeks of
continuous PPI therapy.
RPh may approve a
quantity limit of 30 doses
(not to exceed the GERD
dose in the Table) over
90 days if time is needed
to taper off PPI. Note: No
specific PPI taper
regimen has proven to be
superior. H2RAs may be
helpful during the taper.
Preferred H2RAs are
available without PA.

Oregon Medicaid PA Criteria 186 July 1, 2017
Table. Dosing and Duration of PPI Therapy for OHP Funded Conditions.
Funded OHP Conditions*
Maximum Duration
Maximum Daily Dose
GERD:
Esophageal reflux (K219)
Esophagitis (K200-K210)
8 weeks*
*Treatment beyond 8 weeks is
not funded by OHP.
Dexlansoprazole 30 mg
Dexlansoprazole Solu Tab 30 mg
Esomeprazole 20 mg
Lansoprazole 15 mg
Omeprazole 20 mg
Pantoprazole 40 mg
Rabeprazole 20 mg
H. pylori Infection (B9681)
2 weeks
Dexlansoprazole 60 mg
Dexlansoprazole 30 mg†
Esomeprazole 40 mg
Lansoprazole 60 mg
Omeprazole 40 mg
Pantoprazole 80 mg
Rabeprazole 40 mg
Achalasia and cardiospasm (K220)
Barrett’s esophagus (K22.70; K22.71x)
Duodenal Ulcer (K260-K269)
Dyskinesia of esophagus (K224)
Esophageal hemorrhage (K228)
Gastritis and duodenitis (K2900-K2901; K5281)
Gastroesophageal laceration-hemorrhage
syndrome (K226)
Gastric Ulcer (K250-K259)
Gastrojejunal ulcer (K280-K289)
Malignant mast cell tumors (C962)
Multiple endocrine neoplasia [MEN] type I (E3121)
Neoplasm of uncertain behavior of other and
unspecified endocrine glands (D440; D442; D449)
Peptic ulcer site unspecified (K270-K279)
Perforation of Esophagus (K223)
Stricture & Stenosis of Esophagus (K222)
Zollinger-Ellison (E164)
1 year
*A current list of funded conditions is available at: http://www.oregon.gov/oha/herc/Pages/PrioritizedList.aspx
† Dexlansoprazole SoluTab 30 mg (given as 2 SoluTabs at once) are not recommended for healing of erosive
esophagitis.
P&T / DUR Review: 5/17(KS); 1/16; 5/15; 3/15; 1/13; 2/12; 9/10; 3/10; 12/09; 5/09; 5/02; 2/02; 9/01, 9/98
Implementation: 6/8/16; 2/16; 10/15; 7/15; 4/15; 5/13; 5/12; 1/11; 4/10; 1/10; 9/06, 7/06, 10/04, 3/04

Oregon Medicaid PA Criteria 187 July 1, 2017
Oral/Inhaled Pulmonary Arterial Hypertension Agents
Goals:
Restrict use to patients with pulmonary arterial hypertension (PAH) and World Health
Organization (WHO) Functional Class II-IV symptoms.
Restrict use to conditions funded by the Oregon Health Plan (OHP). Note: erectile dysfunction
is not funded by the OHP.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What is the diagnosis?
Record ICD10 code
2. Is this an OHP-funded diagnosis?
Yes: Go to #3
No: Pass to RPh. Deny; not
funded by the OHP.
3. Is the drug being prescribed by a
pulmonologist or cardiologist?
Yes: Go to #4
No: Pass to RPh. Deny; medical
appropriateness.
4. Is there a diagnosis of pulmonary
arterial hypertension (PAH) (WHO
Group 1)?
Yes: Go to #8
No: Go to #5
5. Is there a diagnosis of chronic
thromboembolic pulmonary
hypertension (WHO Group 4)?
Yes: Go to #6
No: Go to #10
6. Is the request for riociguat
(Adempas
®
)?
Yes: Go to #7
No: Go to #10
7. Is the patient classified as having
World Health Organization (WHO)
Functional Class II-IV symptoms?
Yes: Approve for 12
months
No: Pass to RPh. Deny; medical
appropriateness.
8. Will the prescriber consider a
change to a preferred product?
Yes: Inform prescriber of
preferred alternatives in
No: Go to #9

Oregon Medicaid PA Criteria 188 July 1, 2017
Note: preferred products do not
require PA.
class.
9. Is the patient classified as having
World Health Organization (WHO)
Functional Class II-IV symptoms?
Yes: Approve for 12
months
No: Pass to RPh. Deny; medical
appropriateness.
10. RPh Only: Prescriber must provide
supporting literature for use.
Yes: Approve for length of
treatment.
No: Deny; not funded by the
OHP
P&T Review: 3/16 (AG); 7/14; 3/14; 2/12; 9/10
Implementation: 10/13/16; 5/1/16; 5/14/12; 1/24/12; 1/1/11
Oregon Medicaid PA Criteria 189 July 1, 2017
Injectable Pulmonary Arterial Hypertension Agents (IV/SC)
Goals:
Restrict use to patients with pulmonary arterial hypertension (PAH) and World Health
Organization (WHO) Functional Class III-IV symptoms.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the diagnosis an OHP-funded condition?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP.
3. Will the prescriber consider a change to a
preferred product?
Note: preferred products do not require PA.
Yes: Inform prescriber
of preferred alternatives
in class.
No: Go to #4
4. Is there a diagnosis of pulmonary arterial
hypertension (PAH) (WHO Group 1)?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness.
5. Is the patient classified as having World
Health Organization (WHO) Functional
Class III-IV symptoms?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness.
6. Is the drug being prescribed by a
pulmonologist or a cardiologist?
Yes: Approve for 12
months
No: Pass to RPh. Deny;
medical
appropriateness.
P&T Review: 3/16 (AG); 9/12
Implementation: 10/13/16; 1/1/13
Oregon Medicaid PA Criteria 190 July 1, 2017
Repository Corticotropin Injection
Goal(s):
Restrict use to patient populations in which corticotropin has demonstrated safety and
effectiveness.
Length of Authorization:
4 weeks
Requires PA:
Repository Corticotropin Injection (H.P. Acthar Gel for Injection)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis monotherapy for infantile
spasms in infants and children under 2
years of age?
Yes: Approve up to 4
weeks (2 weeks of
treatment and 2-week
taper)
No: Go to #3
3. Is the diagnosis for acute exacerbation or
relapse of multiple sclerosis?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Has the patient tried and been unable to
tolerate intravenous methylprednisolone or
high-dose oral methylprednisolone?
Yes: Approve up to 5
weeks (3 weeks of
treatment, followed by 2-
week taper).
No: Go to #5

Oregon Medicaid PA Criteria 191 July 1, 2017
Approval Criteria
5. Is the prescription for adjunctive therapy for
short-term administration in corticosteroid-
responsive conditions, including:
The following rheumatic disorders: psoriatic
arthritis, rheumatoid arthritis, juvenile
rheumatoid arthritis or ankylosing
spondylitis;
OR
The following collagen diseases: systemic
lupus erythematosus or systemic
dermatomyositis;
OR
Dermatologic diseases such as erythema
multiforme or Stevens-Johnson syndrome;
OR
Ophthalmic diseases such as keratitis, iritis,
uveitis, optic neuritis, or chorioretinitis;
OR
For the treatment of respiratory diseases,
including symptomatic sarcoidosis or for
treatment of an edematous state?
Yes: Go to #6
No: Pass to RPh. Deny;
medical
appropriateness
6. Is there a contraindication, intolerance, or
therapeutic failure with at least one
intravenous corticosteroid?
Yes: Approve for 6
months.
No: Pass to RPh. Deny;
medical
appropriateness.
P&T Review: 11/16 (DM); 5/13
Implementation: 1/1/17; 1/1/14

Oregon Medicaid PA Criteria 192 July 1, 2017
Repository Corticotropin Injection (Acthar Gel®)
Goal(s):
To ensure appropriate drug use and limit to patient populations in which corticotropin has been
shown to be effective and safe.
Length of Authorization:
4 weeks
Requires PA:
Repository Corticotropin Injection (Acthar Gel
®
)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis monotherapy for infantile
spasms in infants and children under 2
years of age (ICD10 G40821-G40824)?
Yes: Approve up to 4
weeks (2 weeks of
treatment and 2-week
taper)
No: Go to #3
3. Is the diagnosis for acute exacerbation or
relapse of multiple sclerosis (ICD10 G35)?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Has the patient tried and been unable to
tolerate IV methylprednisolone or oral high-
dose methylprednisolone?
Yes: Approve up to 5
weeks (3 weeks of
treatment, followed by 2-
week taper).
No: Go to #5

Oregon Medicaid PA Criteria 193 July 1, 2017
Approval Criteria
5. Is the prescription for adjunctive therapy for
short-term administration in corticosteroid-
responsive conditions, including:
The following rheumatic disorders: psoriatic
arthritis, rheumatoid arthritis, juvenile
rheumatoid arthritis or ankylosing spondylitis
(ICD10 L4054; L4059; M069; M0800; M459;
M3210);
OR
The following collagen diseases: systemic
lupus erythematosus or systemic
deramtomyositis (ICD10 M3210; M3390;
M3320);
OR
Dermatologic diseases such as erythema
multiforme or Stevens-Johnson syndrome
(ICD10 L510; L519; L511; L513);
OR
Ophthalmic diseases such as keratitis, iritis,
uveitis, optic neuritis, or chorioretinitis
(ICD10 H2000; H20019; H20029; H20039;
H20049; H20059; H2013; H209; H20819;
H4040X0; H2023; H20829; H209; H469;
H3093);
OR
For the treatment of respiratory diseases,
including symptomatic sarcoidosis or for
treatment of an edematous state (ICD10
R600; R601; R609)?
Yes: Go to #6
No: Go to #6
6. Is there a contraindication, intolerance, or
therapeutic failure with at least one
intravenous corticosteroid?
Yes: Approve for 6
months.
No: Pass to RPh. Deny;
medical
appropriateness.
P&T Review: 5/30/13 (MH)
Implementation: 5/1/16; 1/1/14

Oregon Medicaid PA Criteria 194 July 1, 2017
Rifaximin (Xifaxan®)
Goal:
Restrict use of rifaximin to OHP-funded conditions and in populations in which the drug has
demonstrated efficacy.
Length of Authorization:
Up to 12 months
Requires PA:
Rifaximin
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the treating diagnosis prevention
or treatment of hepatic
encephalopathy (K7290, K7291)?
Yes: Go to #3
No: Pass to RPh. Deny; not funded by
OHP or for medical appropriateness
3. Is the patient currently managed
with a regularly scheduled daily
regimen of lactulose?
Yes: Go to #5
No: Go to 4
4. Does the patient have a
contraindication to lactulose?
Yes: Go to #5
No: Pass to RPh Deny; medical
appropriateness
Note: studies demonstrate effectiveness
of rifaximin as add-on therapy to
lactulose.
5. Is the patient currently prescribed a
benzodiazepine drug?
Yes: Go to #6
No: Approve for up to 12 months
6. Is the patient tapering off the
benzodiazepine?
Note: tapering process may be
several months
Yes: Approve for
up to 12 months
No: Pass to RPh. Deny; medical
appropriateness
Note: studies explicitly excluded use of
benzodiazepines and benzodiazepine-
like drugs because of their risk for
precipitating an episode of hepatic
encephalopathy.
P&T/DUR Review: 7/15; 5/15 (AG)
Implementation 10/15; 8/15

Oregon Medicaid PA Criteria 195 July 1, 2017
Risperdal
®
Consta
®
Quantity Limit
Goal(s):
To ensure the use of the appropriate billing quantity. This is a quantity initiative, not a clinical
initiative. The vial contains 2 mL. The dispensing pharmacy must submit the quantity as 1 vial
and not 2 mL.
Length of Authorization:
Date of service or 12 months, depending on criteria
Requires PA:
Risperdal
®
Consta
®
Approval Criteria
1. Is the quantity being submitted by the
pharmacy expressed correctly as #
syringes?
Yes: Go to #2
No: Have pharmacy
correct to number of
syringes instead of
number of mL.
2. Is the amount requested above 2 syringes
per 18 days for one of the following
reasons?
Medication lost
Medication dose contaminated
Increase in dose or decrease in dose
Medication stolen
Admission to a long term care facility
Any other reasonable explanation?
Yes: Approve for date of
service only (use
appropriate PA reason)
No: Go to #3
3. Is the pharmacy entering the dose correctly
and is having to dispense more than 2
syringes per 18 days due to the directions
being given on a weekly basis instead of
every other week.
Yes: Approve for 1 year
(use appropriate PA
reason)
Note: This medication
should NOT be denied
for clinical reasons.
P&T Review: 9/16; 5/05
Implementation: 10/13/16; 11/18/04

Oregon Medicaid PA Criteria 196 July 1, 2017
Roflumilast
Goals:
Decrease the number of COPD exacerbations in patients with severe COPD associated with
chronic bronchitis and with a history of exacerbations.
Length of Authorization:
Up to 12 months
Covered Alternatives:
Preferred alternatives listed at http://www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis an OHP-funded diagnosis?
Yes: Go to #3
No: Pass to RPh.
Deny; not covered by
the OHP
3. Does the patient have documented severe
(GOLD 3) or very severe (GOLD 4) COPD?
Yes: Go to #4
No: Pass to RPh. Deny
for medical
appropriateness
4. Does the patient have a diagnosis of chronic
bronchitis (ICD10 J410-J42; J440-J449)?
Yes: Go to #5
No: Pass to RPh. Deny
for medical
appropriateness
5. Does the patient have documented prior
COPD exacerbations?
Yes: Go to #6
No: Pass to RPh. Deny
for medical
appropriateness
6. Does the patient have an active prescription
for a long-acting bronchodilator (long-acting
anticholinergic agent or long-acting beta-
agonist) and inhaled corticosteroid (ICS)?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny;
recommend trial of
preferred long-acting
bronchodilator and ICS
P&T/DUR Review: 9/15 (KS); 5/13; 2/12
Implementation: 10/15; 1/14; 5/12

Oregon Medicaid PA Criteria 197 July 1, 2017
Sacubitril/Valsartan (Entresto™)
Goal(s):
Restrict use of sacubitril/valsartan in populations and at doses in which the drug has
demonstrated efficacy.
Encourage use of beta-blockers with demonstrated evidence of mortality reduction in heart failure
with reduced ejection fraction.
Length of Authorization:
60 days to 12 months
Requires PA:
Sacubitril/valsartan (Entresto™)
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is this a request for continuation of therapy
previously approved by the FFS program?
Yes: Go to Renewal
Criteria
No: Go to #2
2. What diagnosis is being treated?
Record ICD10 code.
3. Does the patient have stable New York
Heart Association Class II or III heart failure
with reduced ejection fraction less than 40%
(LVEF <40%)?
Yes: Go to #4
No: Pass to RPh. Deny;
medical
appropriateness
4. Has the patient tolerated a minimum daily
dose an ACE-inhibitor or ARB listed in Table
1 for at least 30 days?
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness
5. Is the patient currently on a maximally
tolerated dose of carvedilol, sustained-
release metoprolol succinate, or bisoprolol;
and if not, is there a documented intolerance
or contraindication to each of these beta-
blockers?
Note: the above listed beta-blockers have evidence
for mortality reduction in chronic heart failure at
target doses and are recommended by national and
international heart failure guidelines.
1,2
Carvedilol
and metoprolol succinate are preferred agents on
the PDL.
Yes: Approve for up to
60 days
No: Pass to RPh. Deny;
medical
appropriateness

Oregon Medicaid PA Criteria 198 July 1, 2017
Renewal Criteria
1. Is the patient currently taking
sacubitril/valsartan at the target dose of
97/103 mg 2-times daily?
Yes: Approve for up to
12 months
No: Pass to RPh and
go to #2
2. What is the clinical reason the drug has not
been titrated to the target dose of 97/103 mg
2-times daily?
Document rationale and approve for up to 60 days.
Prior authorization required every 60 days until
target dose achieved.
Table 1. Minimum Daily Doses of ACE-inhibitors or ARBs Required.
1,2
ACE-inhibitor
Angiotensin-2 Receptor Blocker (ARB)
Captopril
50 mg TID
Candesartan
32 mg QDay
Enalapril
10 mg BID
Losartan
150 mg QDay
Lisinopril
20 mg QDay
Valsartan
160 mg BID
Ramipril
5 mg BID
Trandolapril
4 mg QDay
Abbreviations: BID = twice daily; QDay = once daily; mg = milligrams; TID = three times daily.
Notes:
Patients must achieve a minimum daily dose of one of the drugs listed for at least 30 days in order to improve chances of tolerability
to the target maintenance dose of sacubitril/valsartan 97/103 mg 2-times daily.
3
Valsartan formulated in the target maintenance dose of sacubitril valsartan 97/103 mg 2-times daily is bioequivalent to valsartan
160 mg 2-times daily.
4
ACE-inhibitors and ARBs listed have demonstrated efficacy in heart failure with or without myocardial infarction.
1,2
Target daily doses of other ACE-inhibitors and ARBs for heart failure have not been established.
1,2
It is advised that patients previously on an ACE-inhibitor have a 36-hour washout period before initiation of sacubitril/valsartan to
reduce risk of angioedema.
3,4
References:
1. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American
College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol.
2013;62(16):e147-239. doi: 10.1016/j.jacc.2013.05.019.
2. McMurray J, Adamopoulos S, Anker S, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure
2012. European Journal of Heart Failure. 2012;14:803-869. doi:10.1093/eurjhf/hfs105.
3. McMurray J, Packer M, Desai A, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Eng J Med.
2014;371:993-1004. doi:10.1056/NEJMoa1409077.
4. ENTRESTO (sacubitril and valsartan) [Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals, July 2015.
P&T / DUR Review: 05/17(DM), 09/15
Implementation: 10/13/16; 10/1/15

Oregon Medicaid PA Criteria 199 July 1, 2017
Sapropterin
Goal(s):
Promote safe and cost effective therapy for the treatment of phenylketonuria.
Length of Authorization:
Initial: 1 to 2 months; Renewal: 1 year
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the request for renewal of therapy
previously approved by the FFS system?
Yes: Go to Renewal
Criteria
No: Go to #3
3. Is the drug prescribed by or in
consultation with a specialist in metabolic
disorders?
Yes: Go to #4
No: Pass to RPh. Deny;
medical appropriateness
4. Is the diagnosis tetrahydrobiopterin-
(BH4-) responsive phenylketonuria?
Yes: Go to #5
No: Pass to RPh. Deny;
medical appropriateness
5. Is the patient currently compliant with a
Phe-restricted diet and unable to achieve
target blood phenylalanine level?
Yes: Go to #6
No: Pass to RPh. Deny
and recommend Phe-
restricted diet.
6. Is the patient’s baseline blood
phenylalanine level provided in the
request and above the target range (see
Clinical Notes)?
Yes: Approve for 2
months if initial dose is 5-
10 mg/kg/day (to allow for
titration to 20 mg/kg/day).
Approve for 1 month if
initial dose is 20
mg/kg/day (adults and
children).
No: Request information
from provider.
Renewal Criteria
1. Did the patient meet the target
phenylalanine level set by the specialist (see
Clinical Notes)?
Yes: Go to #2
No: Pass to RPh. Deny
for lack of treatment
response.
2. Is the patient remaining compliant with the
Phe-restricted diet?
Yes: Approve for up to
12 months
No: Pass to RPh. Deny
and recommend Phe-
restricted diet.

Oregon Medicaid PA Criteria 200 July 1, 2017
Target blood phenylalanine levels in the range of 120-360 µmol/L for patients in all age ranges.
1
In addition to the recommended Phe concentrations, a 30% or more reduction in blood Phe is often
considered a clinically significant change from baseline and should occur after the initial trial.
2
If not,
the patient is a nonresponder and will not benefit from sapropterin therapy.
Doses above 20 mg/kg/day have not been studied in clinical trials.
References:
1. Vockley J, Andersson HC, Antshel KM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management
guideline. Genet Med. 2014;16(2):188-200. doi:10.1038/gim.2013.157
2. Blau N., Belanger-Quintana A., Demirkol M. Optimizing the use of sapropterin (BH
4
) in the management of
phenylketonuria. Molecular Genetics and Metabolism 2009;96:158-163.
P&T Review: 5/16 (DM); 11/13; 9/13; 7/13
Implementation: 8/16; 1/1/14

Oregon Medicaid PA Criteria 201 July 1, 2017
Sedatives
Goal(s):
Restrict use of sedatives to OHP-funded conditions. Treatment of uncomplicated insomnia is not
funded; insomnia contributing to covered co-morbid conditions is funded.
Prevent concomitant use of sedatives, benzodiazepines, and opioids.
Restrict long-term sedative use to due to insufficient evidence and to limit adverse effects.
Limit zolpidem use the maximum FDA recommended daily dose based on gender.
Length of Authorization:
Up to 12 months (criteria specific)
Requires PA:
All sedatives
Concomitant use of more than one benzodiazepine, more than one non-benzodiazepine sedative,
or the combination of a benzodiazepine and non-benzodiazepine sedative in the prior 30 days.
Sedatives that exceed a total quantity of 30 doses within 60 days
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Zolpidem Daily Quantity Limits
Generic
Brand
Max Daily Dose
Male
Female
Zolpidem IR
Ambien
10 mg
5 mg
Zolpidem ER
Ambien CR
12.5 mg
6.25 mg
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the request for zolpidem at a higher dose
than listed in the quantity limit chart?
Yes: Pass to RPh.
Deny; medical
appropriateness.
No: Go to #3
3. Is the request for a non-preferred product
and will the prescriber consider a change to
a preferred product?
Message: Preferred products are evidence
based and reviewed for comparative
effectiveness and safety by the P&T
Committee.
Yes: Inform prescriber
of preferred alternatives
in class.
No: Go to #4
4. Does patient have diagnosis of insomnia
with obstructive sleep apnea?
Yes: Go to #5
No: Go to #6

Oregon Medicaid PA Criteria 202 July 1, 2017
Approval Criteria
5. Is patient on CPAP?
Yes: Approve for up to
12 months.
No: Pass to RPh. Deny;
medical
appropriateness.
Sedative/hypnotics, due
to depressant effect,
are contraindicated.
6. Is the patient being treated for co-morbid:
Depression;
Anxiety or panic disorder; or
Bipolar disorder?
AND
Is there an existing claim history for
treatment of the co-morbid condition (e.g.,
antidepressant, lithium, lamotrigine,
antipsychotic, or other appropriate mental
health drug)?
Yes: Approve for up to
12 months.
No: Go to #7
7. Has the patient been treated with another
non-benzodiazepine sedative,
benzodiazepine, or opioid within the past 30
days?
Yes: Go to #8
No: Pass to RPh; Go to
#9
8. Is this a switch in sedative therapy due to
intolerance, allergy or ineffectiveness?
Yes: Document reason
for switch and approve
duplication for 30 days.
No: Pass to RPh. Deny;
medical
appropriateness.
9. RPh only: Is diagnosis being treated a
funded condition and is there medical
evidence of benefit for the prescribed
sedative?
Funded: Document
supporting literature and
approve up to 6 months
with subsequent
approvals dependent on
follow-up and
documented response.
Not Funded: Go to #10
10. RPh only: Is this a request for continuation
therapy for a patient with a history of chronic
benzodiazepine use where discontinuation
would be difficult or unadvisable?
Yes: Document length
of treatment and last
follow-up date. Approve
for up to 12 months.
No: Deny; medical
appropriateness
P&T/DUR Review: 3/17 (SS); 11/20/14, 3/27/14, 5/18/06, 2/23/06, 11/10/05, 9/15/05, 2/24/04, 2/5/02, 9/7/01
Implementation: TBD; 1/1/15, 7/1/14; 1/1/07, 7/1/06, 11/15/05

Oregon Medicaid PA Criteria 203 July 1, 2017
Sodium-Glucose Cotransporter-2 Inhibitors (SGLT-2 Inhibitors)
Goal(s):
Promote cost-effective and safe step-therapy for management of type 2 diabetes mellitus (T2DM).
Length of Authorization:
Up to 6 months
Requires PA:
All SGLT-2 inhibitors
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. Is this a request for renewal of a previously
approved prior authorization?
Yes: Go the
Renewal Criteria
No: Go to #2
2. What diagnosis is being treated?
Record ICD10 code
3. Does the patient have a diagnosis of T2DM?
Yes: Go to #4
No: Pass to RPh. Deny;
medical appropriateness
4. Has the patient tried and failed metformin
and a sulfonylurea, have contraindications
to these treatments or is requesting a SGLT-
2 inhibitor to be used with metformin and a
sulfonylurea?
(document contraindication, if any)
Yes: Go to #5
No: Pass to RPh. Deny and
recommend trial of metformin
or sulfonylurea. See below
for metformin titration
schedule.
5. Is the request for the following treatments
(including combination products) with an
associated estimated glomerular filtration
rate (eGFR):
Canagliflozin and eGFR <45 mL/min/
1.73 m
2
, or
Empagliflozin and eGFR <45 mL/min/
1.73 m
2
, or
Dapagliflozin and eGFR <60 mL/min/
1.73 m
2
?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Go to #6

Oregon Medicaid PA Criteria 204 July 1, 2017
Approval Criteria
6. Has the patient tried and failed (unable to
maintain goal A1c) all of the following drugs,
or have contraindications to all of these
drugs?
1. Insulin
2. Thiazolidinedione
3. DPP-4 inhibitor
4. GLP-1 receptor agonist
5. Amylin analog
Yes: Approve for
up to 6 months
No: Pass to RPh. Deny and
require a trial of insulin,
thiazolidinedione, DPP-4
inhibitor, GLP-1 agonist, and
amylin analog.
Renewal Criteria
Is the request for the following treatments
(including combination products) with an
associated estimated glomerular filtration rate
(eGFR):
Canagliflozin and eGFR <45 mL/min/
1.73 m
2
, or
Empagliflozin and eGFR <45 mL/min/
1.73 m
2
, or
Dapagliflozin and eGFR <60 mL/min/
1.73 m
2
?
Yes: Pass to RPh.
Deny; medical
appropriateness
No: Approve for up to 6
months
Initiating Metformin
1. Begin with low-dose metformin (500 mg) taken once or twice per day with meals (breakfast and/or dinner) or 850 mg once per
day.
2. After 5-7 days, if gastrointestinal side effects have not occurred, advance dose to 850 mg, or two 500 mg tablets, twice per day
(medication to be taken before breakfast and/or dinner).
3. If gastrointestinal side effects appear with increasing doses, decrease to previous lower dose and try to advance the dose at a later
time.
4. The maximum effective dose can be up to 1,000 mg twice per day but is often 850 mg twice per day. Modestly greater
effectiveness has been observed with doses up to about 2,500 mg/day. Gastrointestinal side effects may limit the dose that can be
used.
Nathan, et al. Medical management of hyperglycemia in Type 2 Diabetes: a consensus algorithm for the initiation and adjustment of
therapy. Diabetes Care. 2008; 31;1-11.
P&T Review: 9/16 (KS); 3/16; 9/15; 1/15; 9/14; 9/13
Implementation: 10/13/16; 2/3/15; 1/1/14

Oregon Medicaid PA Criteria 205 July 1, 2017
Skeletal Muscle Relaxants
Goal(s):
Cover non-preferred drugs only for funded conditions.
Restrict carisoprodol to short-term use due to lack of long-term studies to assess safety or
efficacy and high potential for abuse.
Length of Authorization:
Up to 3 - 6 months
Requires PA:
Non-preferred agents
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis funded by the Oregon
Health Plan?
Yes: Go to #3
No: Pass to RPh. Deny;
not funded by the OHP
3. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require PA
Preferred products are evidence-based
reviewed for comparative effectiveness
and safety by the Pharmacy and
Therapeutics (P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class
No: Go to #4
4. Is drug requested carisoprodol?
Yes: Go to #5
No: Approve for up to 3
months
5. Has an opioid been prescribed within the
past 30 days?
Yes: Deny; medical
appropriateness
No: Go to #6

Oregon Medicaid PA Criteria 206 July 1, 2017
Approval Criteria
6. Does total quantity of carisoprodol exceed
56 tablets in 90 days?
From claims, document product, dose,
directions, and amount used during last 90
days.
Yes: Go to #7
No: Approve for up to 3
months
7. Does patient have a terminal illness (e.g.
metastatic cancer, end stage Parkinson’s
disease, ALS)?
Yes: Approve for 6
months.
No: Pass to RPh. Go to
#8
8. Pharmacist’s statement:
Carisoprodol cannot be approved for
long term usage.
Patients are limited to 56 tablets in a 90
day period.
It is recommended that the patient
undergo a “taper” of the carisoprodol
product of which a supply may be
authorized for this to occur.
The amount and length of taper
depends upon the patient’s condition.
Does the patient meet one or more of
the following:
o >65 years of age; or
o renal failure; or
o hepatic failure; or
o take > 1400 mg per day?
Yes: Document reason
and approve long taper:
Authorize 18 tablets
Reduce dose over 9
days
350 mg TID X 3
days, then
350 mg BID X 3
days, then
350 mg daily x 3
days then evaluate
No: Approve short
taper:
Authorize 10 tablets
Reduce dose over 4
days
350 mg TID x 1 day,
then
350 mg BID x 2
days, then
350 mg daily x1
day, then evaluate
P&T Review: 3/17 (DM); 3/17; 11/14; 9/09; 2/06; 2/04; 11/01; 2/01; 9/00; 5/00; 2/00
Implementation: 4/1/17; 1/1/15, 1/1/14, 1/1/10, 11/18/04

Oregon Medicaid PA Criteria 207 July 1, 2017
Smoking Cessation
Goal(s):
Promote use that is consistent with National Guidelines and medical evidence.
Promote use of high value products
Length of Authorization:
3-6 months
Requires PA:
Non-preferred drugs
Nicotine replacement therapy (NRT) for more than 6 months in the absence of behavioral
counseling
Varenicline treatment for more than 12 weeks
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Is the diagnosis for tobacco dependence
(ICD10 F17200)?
Yes: Go to #3
No: Pass to RPh. Deny;
medical
appropriateness
3. Is the request for a preferred NRT product?
Yes: Go to #5
No: Go to #4
4. Is the request for varenicline?
Yes: Go to #5
No: Go to #7
5. Has patient quit?
Yes: Approve NRT for 6
additional months or
approve varenicline for
12 additional weeks
No: Go to #6
6. Is the patient enrolled in a smoking
cessation behavioral counseling program
[e.g. Quit Line at: 800-QUIT-NOW (800-784-
8669)].
Yes: Approve NRT for 6
additional months or
approve varenicline for
12 additional weeks
No: Pass to RPh. Deny;
medical
appropriateness

Oregon Medicaid PA Criteria 208 July 1, 2017
Approval Criteria
7. Will the prescriber change to a preferred
product?
Message:
• Preferred products do not require a PA for
initial treatment.
• Preferred products are evidence-based
reviewed for comparative effectiveness and
safety by the Pharmacy and Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class
No: Approve treatment
for up to 6 months
P&T Review: 7/16 (MH); 4/12
Implementation: 8/16, 7/23/12

Oregon Medicaid PA Criteria 209 July 1, 2017
Tesamorelin (Egrifta
®
)
Goal(s):
Restrict to indications funded by the OHP and supported by medical literature.
Length of Authorization:
Up to 12 months
Requires PA:
Tesamorelin (Egrifta
®
)
Covered Alternatives:
No preferred alternatives
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Is the indicated treatment for reduction of
excess abdominal fat in HIV-infected
patients with lipodystrophy (ICD10 E881)?
Yes: Pass to RPh.
Deny; not funded by the
OHP.
No: Go to #3
3. RPh only: All other diagnoses must be evaluated as to funding level on OHP and evidence for
must be provided by the prescriber that supports use. Evidence will be forwarded to Oregon
DMAP for consideration.
P&T/DUR Review: 9/15 (AG); 4/12
Implementation: 10/15; 7/12

Oregon Medicaid PA Criteria 210 July 1, 2017
Testosterone
Goal(s):
Restrict use to medically appropriate conditions funded under the Oregon Health Plan (use for
sexual dysfunction or body-building is not covered)
Length of Authorization:
Up to 12 months
Requires PA:
All topical testosterone products and non-preferred injectable testosterone products in adults
All testosterone products in pediatric patients <18 years of age
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code.
2. Does the diagnosis for the medication
requested include any of the following?
Testicular Hypofunction; or
Hypopituitarism and related disorders; or
AIDS-related cachexia?
Yes: Go to #5
No: Go to #3
3. Is the medication requested for gender
dysphoria (ICD10 F642, F641)?
Yes: Go to #4
No: Go to #6
4. Have all of the following criteria been met?
Patient has the capacity to make fully
informed decisions and to give consent
for treatment; and
If patient <18 years of age, the prescriber
is a pediatric endocrinologist; and
The prescriber agrees criteria in the
Guideline Notes on the OHP List of
Prioritized Services have been met.
Yes: Go to #5
No: Pass to RPh. Deny;
medical
appropriateness

Oregon Medicaid PA Criteria 211 July 1, 2017
Approval Criteria
5. Will the prescriber consider a change to a
preferred product?
Message:
Preferred products do not require a
co-pay.
Preferred products are evidence-
based reviewed for comparative
effectiveness and safety by the
Oregon Pharmacy & Therapeutics
(P&T) Committee.
Yes: Inform prescriber
of covered alternatives
in class and approve for
up to 12 months.
No: Approve for up to
12 months.
6. RPh only: all other indications need to be
evaluated to see if funded under the OHP.
If funded and prescriber
provides supporting
literature: Approve for up
to 12 months.
If not funded: Deny; not
funded by the OHP
P&T Review: 11/15 (KS); 2/12; 9/10; 2/06; 2/01; 9/00
Implementation: 5/1/16; 1/1/16; 7/31/14; 5/14/12, 1/24/12, 1/1/11, 9/1/06

Oregon Medicaid PA Criteria 212 July 1, 2017
Topical Antipsoriasis Drugs
Goal(s):
Restrict topical antipsoriasis drugs only for funded OHP diagnoses. Moderate/Severe psoriasis
treatments are funded on the OHP. Treatments for mild psoriasis (L400-404,L408-418, L448),
seborrheic dermatitis (L2083,L210-219,L303), keroderma (L110, L83, L850-852, L870-872,
L900-902, L906, L940, L943) and other hypertrophic and atrophic conditions of skin (L119,
L572, L574, L664, L908-909, L918-919, L922, L985) are not funded.
Length of Authorization:
Up to 12 months
Requires PA:
Non-preferred drugs
TC = 92 and HIC = L1A, L5F, L9D, T0A
Covered Alternatives:
Preferred alternatives listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD 10 code.
2. Is the diagnosis for seborrheic
dermatitis (L2083,L210-219,L303 ),
keroderma (L110, L83, L850-852,
L870-872, L900-902, L906, L940,
L943 ) or other hypertrophic and
atrophic conditions of skin (L119,
L572, L574, L664, L908-909, L918-
919, L922, L985 )?
Yes: Pass to RPh; deny, not
funded by the OHP.
No: Go to #3
3. Is the diagnosis Psoriasis?
( ICD-10 L400-404,L408-418,, L448)
Yes: Go to #4
No: Go to #7
4. Is the Psoriasis Moderate/Severe?
Defined as:
At least 10% body surface area
involved or with functional
impairment?
Hand, foot or mucous membrane
involvement
Yes: Go to #5
No: Pass to RPh; deny,
not funded by the OHP.
5. Is the product requested preferred?
Yes: Approve for length of
treatment; maximum 1 year.
No: Go to #6

Oregon Medicaid PA Criteria 213 July 1, 2017
Approval Criteria
6. Will the prescriber consider a change
to a preferred product?
Message:
Preferred products are evidence-
based reviewed for comparative
effectiveness & safety by the
Pharmacy and Therapeutics (P&T)
Committee.
Yes: Inform provider of
preferred alternatives.
Approve for length of
treatment; maximum 1 year.
No: Approve for length
of treatment; maximum
1 year.
7. RPH only:
All other indications need to be
evaluated as to whether they are
funded by the OHP.
If funded, or clinic provides
supporting literature: approve
for length of treatment.
If not funded: Deny, not
funded by the OHP.
P&T/DUR Review: 7/15; 1/15; 09/10; 9/09; 3/09; 5/07; 2/06
Implementation: 10/15; 8/15; 9/13; 6/12; 9/10; 1/10; 7/09; 6/07; 9/06
Oregon Medicaid PA Criteria 214 July 1, 2017
Topiramate
Goal(s):
Approve topiramate only for funded diagnoses which are supported by the medical literature (e.g.
epilepsy and migraine prophylaxis).
Length of Authorization:
90 days to lifetime
Requires PA:
Non-preferred topiramate products
Covered Alternatives:
Current PMPDP preferred drug list per OAR 410-121-0030 at www.orpdl.org
Searchable site for Oregon FFS Drug Class listed at www.orpdl.org/drugs/
Approval Criteria
1. What diagnosis is being treated?
Record ICD10 code
2. Does the patient have diagnosis of
epilepsy?
Yes: Approve for lifetime
(until 12-31-2036)
No: Go to #3
3. Does the patient have a diagnosis of
migraine?
Yes: Approve for 90 days
with subsequent approvals
dependent on documented
positive response for
lifetime*
No: Go to #4
4. Does the patient have a diagnosis of
bipolar affective disorder or
schizoaffective disorder?
Yes: Go to #5
No: Go to #6
5. Has the patient tried or are they
contraindicated to at least two of the
following drugs?
Lithium
Valproate and derivatives
Lamotrigine
Carbamazepine
Atypical antipsychotic
Document drugs tried or contraindications.
Yes: Approve for 90 days
with subsequent approvals
dependent on documented
positive response for
lifetime approval.*
No: Pass to RPh;
Deny; medical
appropriateness.
Recommend trial of 2
covered alternatives.
6. Is the patient using the medication for
weight loss? (Obesity ICD10 E669;
E6601)?
Yes: Pass to RPh. Deny;
not funded by the OHP
No: Pass to RPh. Go
to #7

Oregon Medicaid PA Criteria 215 July 1, 2017
Approval Criteria
7. All other indications need to be evaluated
for appropriateness:
Neuropathic pain
Post-Traumatic Stress Disorder (PTSD)
Substance abuse
Use is off-label: Deny; medical appropriateness.
Other treatments should be tried as appropriate.
Use is unfunded: Deny; not funded by the OHP.
If clinically warranted: Deny; medical
appropriateness. Use clinical judgment to approve
for 1 month to allow time for appeal.
MESSAGE: “Although the request has been denied
for long-term use because it is considered medically
inappropriate, it has also been APPROVED for one
month to allow time for appeal.”
P&T Review: 3/17 (DM); 7/16; 3/15; 2/12; 9/07; 11/07
Implementation: 4/18/15; 5/12, 1/12
