
Acta Physiologica. 2022;00:e13777.
|
1 of 20
https://doi.org/10.1111/apha.13777
wileyonlinelibrary.com/journal/apha
Received: 29 May 2021
|
Revised: 27 September 2021
|
Accepted: 1 January 2022
DOI: 10.1111/apha.13777
REGULAR PAPER
A novel K
+
- dependent Na
+
uptake mechanism during low
pH exposure in adult zebrafish (Danio rerio): New tricks for
old dogma
Alexander M.Clifford
1,2
|
MartinTresguerres
2
|
Greg G.Goss
3
|
Chris M.Wood
1
© 2022 Scandinavian Physiological Society. Published by John Wiley & Sons Ltd
1
Department of Zoology, University of
British Columbia, Vancouver, British
Columbia, Canada
2
Marine Biology Research Division,
Scripps Institution of Oceanography,
University of California San Diego, La
Jolla, California, USA
3
Department of Biological Sciences,
University of Alberta, Edmonton,
Alberta, Canada
Correspondence
Alexander M. Clifford, Scripps
Institution of Oceanography, University
of California San Diego, 8750 Biological
Grade, Hubbs Hall 3120, La Jolla, CA
92037.
Email: [email protected]
Funding information
AMC was supported by a Natural
Sciences and Engineering Research
Council (NSERC) Discovery
grant awarded to CMW (RGPIN-
2017- 03843), and a Scripps Institution
Oceanography Postdoctoral Research
Scholar Fellowship. MT provided SIO
discretionary funds and was funded by
the National Science Foundation (IOS#
1754994). GGG was funded by NSERC
(RGPIN- 2016- 04678).
Abstract
Aim: To determine whether Na
+
uptake in adult zebrafish (Danio rerio) exposed
to acidic water adheres to traditional models reliant on Na
+
/H
+
Exchangers
(NHEs), Na
+
channels and Na
+
/Cl
−
co- transporters (NCCs) or if it occurs through
a novel mechanism.
Methods: Zebrafish were exposed to control (pH 8.0) or acidic (pH 4.0) water
for 0- 12hours during which
22
Na
+
uptake (
J
Na
in
), ammonia excretion, net acidic
equivalent flux and net K
+
flux (
J
H
net
) were measured. The involvement of
NHEs, Na
+
channels, NCCs, K
+
- channels and K
+
- dependent Na
+
/Ca
2+
exchang-
ers (NCKXs) was evaluated by exposure to Cl
−
- free or elevated [K
+
] water, or to
pharmacological inhibitors. The presence of NCKXs in gill was examined using
RT- PCR.
Results:
J
Na
in
was strongly attenuated by acid exposure, but gradually recov-
ered to control rates. The systematic elimination of each of the traditional models
led us to consider K
+
as a counter substrate for Na
+
uptake during acid expo-
sure. Indeed, elevated environmental [K
+
] inhibited
J
Na
in
during acid exposure
in a concentration- dependent manner, with near- complete inhibition at 10mM.
Moreover,
J
H
net
loss increased approximately fourfold at 8- 10hours of acid ex-
posure which correlated with recovered
J
Na
in
in 1:1 fashion, and both
J
Na
in
and
J
H
net
were sensitive to tetraethylammonium (TEA) during acid exposure.
Zebrafish gills expressed mRNA coding for six NCKX isoforms.
Conclusions: During acid exposure, zebrafish engage a novel Na
+
uptake mech-
anism that utilizes the outwardly directed K
+
gradient as a counter- substrate for
Na
+
and is sensitive to TEA. NKCXs are promising candidates to mediate this
K
+
- dependent Na
+
uptake, opening new research avenues about Na
+
uptake in
zebrafish and other acid- tolerant aquatic species.
KEYWORDS
ionoregulation, low pH, Na
+
/Ca
2+
- K
+
exchanger, Na
+
/H
+
exchanger, Na
+
- C l
−
cotransporter,
sodium uptake

2 of 20
|
CLIFFORD et al.
1
|
INTRODUCTION
Freshwater teleosts are faced with the challenge of dif-
fusive ion loss to their hypo- osmotic surroundings and
thus actively take up Na
+
from the environment. The
current dogma for freshwater fish gills proposes three
Na
+
uptake mechanisms within ion transporting cells
(ionocytes): (a) August Krogh's classic apical Na
+
/H
+
(
NH
4
+
) exchange,
1– 3
(Figure 1A) mediated by Na
+
/H
+
exchangers (NHEs) and possibly augmented by outward
transport of NH
3
by Rhesus (Rh) glycoproteins,
4– 7
(b)
uptake through, as of yet unidentified, apical Na
+
chan-
nel(s) (Figure1B) or related acid- sensing ion channel(s)
(ASICs)
8,9
electrogenically coupled to apical H
+
excre-
tion via V- H
+
- ATPase (VHA),
10– 12
and more recently (c)
co- transport of Na
+
and Cl
−
via Na
+
/Cl
−
co- transporters
(NCCs; Figure1C).
13
These molecular mechanisms are
analogous to apical Na
+
- reabsorption mechanisms in
the mammalian kidney where roughly two- thirds of
Na
+
reabsorption occurs by proximal tubule NHEs and
the remainder is mediated by NCCs and epithelial Na
+
channels (ENaCs) in the distal convoluted tubules and
collecting ducts respectively.
14– 16
Abundant evidence suggests that Na
+
uptake via NHE
is the prevalent mechanism in freshwater teleosts
17– 19
;
however, uptake solely via NHE relies on thermodynam-
ically favourable conditions.
20
The operational direction
of NHE is fundamentally dictated by environmental and
intra- ionocyte concentration gradients of Na
+
and H
+
,
such that Na
+
uptake is favoured only when
At low environmental [Na
+
] or pH (ie high [H
+
]), NHE
will function in the direction of Na
+
excretion, to the det-
riment of Na
+
homeostasis.
10,20
However, many freshwater
fishes can still live in low pH and/or low [Na
+
] water where
NHE should not function. For example wild zebrafish (Danio
rerio) have been observed in shallow streams with pH<6.0,
21
and their natural habitat includes stagnant ponds and rice
paddies that can be even more acidic (as low as pH 3.5) be-
cause of acidic soils or agricultural runoff.
22– 26
Furthermore,
zebrafish are known to aggregate in very dense shoals, which
likely results in additional acidification.
27
Indeed, zebrafish
are quite tolerant of acidic environments, and capable of
long- term (>2weeks) survival in waters as low as pH 4.0.
28
Stimulations of Na
+
uptake by larval zebrafish in response
to acid exposure have been reported,
29,30
suggesting the in-
volvement of mechanisms other than NHE.
One proposed solution to overcoming the thermody-
namic constraints on Na
+
uptake by NHE at low external
pH is by forming a functional metabolon with Rhcg (Rh
glycoprotein type c; a purported NH
3
channel
31
), whereby
Rhcg strips H
+
from
NH
4
+
and transports NH
3
across
the membrane, thereby generating a H
+
driving gradient
powering NHE in the Na
+
uptake direction (Figure1A).
Once outside, NH
3
is re- protonated to
NH
4
+
, thus main-
taining the outwardly directed NH
3
gradient while simul-
taneously raising the local boundary layer pH so that NHE
function in the Na
+
uptake direction is further favoured.
4
In support of this hypothesis, translational knockdown of
either Rhcg1 or NHE3b in larval zebrafish resulted in an
attenuation of stimulated Na
+
uptake in acid- reared ze-
brafish.
29
However, it remains unclear if the NHE/Rhcg
metabolon could function at extremely low pHs, or even
if it is functional in adult zebrafish.
(1)
[Na
+
]
i
[Na
+
]
o
<
[H
+
]
i
[H
+
]
o
FIGURE Putative models for Na
+
uptake in freshwater fishes. (A) August Krogh's classic apical Na
+
/H
+
(
NH
4
+
) exchange mediated
by Na
+
/H
+
Exchangers (NHEs), possibly in combination with Rhesus (Rh) glycoproteins, (B) apical Na
+
channels and/or acid- sensing ion
channels (ASIC) electrogenically coupled to apical proton excretion via V- H
+
- ATPase (VHA), (C) coupled uptake with Cl
−
via Na
+
/Cl
−
co-
transporters (NCC)
1+(
$SLFDO1D
+
1+
H[FKDQJH
%ORRG :DWHU
*LOOLRQRF\WH
6RGLXPFKDQQHO$6,&
1D
FKDQQHOFRXSOHGWRDFWLYH+
H[FUHWLRQ
%ORRG :DWHU
*LOOLRQRF\WH
1&&
1D
DQG&,
FRWUDQVSRUW
%ORRG :DWHU
*LOOLRQRF\WH
1D
&,
+
1+
1+
1+
5KFJ
1
1D
FKDQQHO
$6,&
.
1.$
.
1.$
.
1.$
1D
+
1
1+(
H1%&
1D
&,
&
1
1&&
$73
$73
$73
9+$
$73
&$
+&2
&2
&
&,
FKDQQHO
(A)(B) (C)
1D1D
1D
1D

|
3 of 20
CLIFFORD et al.
In an alternative mechanism, Na
+
uptake in adult ze-
brafish and rainbow trout (Oncorhynchus mykiss) held
in very low (<0.1mM) environmental [Na
+
] seems to be
mediated primarily by ASICs electrogenically coupled to
apical proton excretion via VHA, rather than via NHEs.
In both fish species, amiloride- insensitive Na
+
uptake was
inhibited by the ASIC- inhibitor DAPI (4′,6- diamidino- 2-
phenylindole),
8,9
and in zebrafish, Na
+
uptake persisted
despite NHE3b knockout via CRISPr/Cas9 deletion.
32
However, it is not known whether this mechanism is also
functional during exposure to low pH conditions.
Finally, uptake of Na
+
by zebrafish during acid exposure
may be mediated by apical Na
+
/Cl
−
cotransporters (NCCs).
The supporting evidence includes an increased abundance
of gill NCC cells and decreased expression of nhe3b/NHE3b
following exposure of adult zebrafish to low pH environ-
ments (2- 7 days). In addition, zebrafish larvae exposed to
similar conditions demonstrated an increased abundance of
skin NCC cells, enlarged NCC cells and increased ncc mRNA
expression.
33
In another study, zebrafish larvae pre- exposed
to pH 4.0 for 2hours demonstrated increased Na
+
and Cl
−
influx upon return to circumneutral pH. The uptake of each
ion was attenuated when the other ion was omitted from the
water (ie Cl
–
- free and Na
+
- free conditions respectively) as
well as upon NCC morpholino knockdown; however, VHA
knockdown had no effect.
13
A major caveat is that these flux
measurements were performed in circumneutral pH water,
and therefore evaluated the role of NCC during recovery
from acute acid exposure and not necessarily the mecha-
nism responsible for Na
+
uptake during exposure to acidic
conditions. Moreover, in low [Na
+
] trials, removal of water
Cl
−
(to inhibit potential rescue by a putative NCC mecha-
nism) combined with VHA morpholino knockdown in the
NHE3b knockout zebrafish all failed to reduce Na
+
uptake.
32
Finally, in the proposed model, both [Na
+
] and [Cl
−
] in the
water are multiple orders of magnitude lower than nominal
intracellular concentrations, raising questions about how
NCC transport could be energized. These observations point
to a novel, as of yet undescribed mechanism for Na
+
uptake
in zebrafish in very low [Na
+
] and/or very low pH environ-
ments and in this lies the impetus for the current study.
Our goal was to characterize the acid- inducible Na
+
uptake mechanism in zebrafish by analysis of the re-
covery of Na
+
uptake during continued acid exposure.
We hypothesized that acute exposure to low pH (pH
4.0) conditions would inhibit NHE function because
of adverse ion motive gradients.
20
Radio- labelled
22
Na
was used to measure the return of unidirectional Na
+
uptake flux rates (
J
Na
in
) during exposure, allowing us to
characterize the upregulation of alternate Na
+
uptake
mechanisms. Through a series of flux studies utilizing
putative drug inhibitors (Table 1), ion- replacement,
and kinetic analyses, we ruled out contributions from
the previously proposed Na
+
uptake mechanisms, and
TABLE List of inhibitors and their putative targets
Drug IUPAC name [Drug] Target notes References
Amiloride 3,5- diamino- 6- chloro- N-
(diaminomethylene)
pyrazine- 2- carboxamide
200µM NHE, ENaC, ASIC 9,34,35
DAPI 2- (4- Amidinophenyl)- 1H- indole- 6-
carboxamidine
20µM ASIC, possibly NHE2 8,9,36,37
EIPA 5- (N- Ethyl- N- isopropyl)amiloride 50µM NHE 9,29,34,37,38
Phenamil 3,5- Diamino- 6- chloro- N- (N-
phenylcarbamimidoyl)- 2-
pyrazinecarboxamide
50µM ENaC 34,39– 41
Bumetanide 3- butylamino- 4- phenoxy- 5- sulfamoyl-
benzoic acid
100µM NKCC 37,42
Hydrochlorothiazide 6- chloro- 1,1- dioxo- 3,4- dihydro- 2H- 1,2,4-
benzothiadiazine- 7- sulfonamide
100µM NCC 37,43
Metolazone 7- chloro- 2- methyl- 4- oxo- 3- o- tolyl- 1,2,3,4-
tetrahydroquinazoline- 6- sulfonamide
100µM NCC 44– 46
Acetazolamide 5- acetamido- 1,3,4- thiadiazole- 2-
sulfonamide
100µM CA 10,47
Barium BaCl
2
10mM Broad spectrum K
+
channel inhibitor 48– 51
4- Aminopyridine Pyridin- 4- amine 500µM Kv1 channels
Ca
2+
- activated K
+
channels
52
Tetraethylammonium tetraethylazanium 1mM K
+
channels (Ca
2+
activated, Voltage
gated), NKA, NCKX
53– 58

4 of 20
|
CLIFFORD et al.
uncovered evidence for a thus far unreported Na
+
up-
take mechanism that is electroneutrally linked to out-
ward K
+
movement. This newly identified Na
+
uptake
mechanism operates to rescue Na
+
uptake during expo-
sure to low environmental pH.
2
|
RESULTS
2.1
|
Series 1: Time- course dynamics of
zebrafish ion- regulatory status during acid
exposure
Zebrafish were exposed to either control (pH ~8.0) or acid
(pH 4.0) conditions for up to 12hours while ion flux com-
ponents were characterized intermittently throughout; pH
4.0 was chosen for the acid exposure based on range- finder
tests (see Section 4; Series 1). In zebrafish exposed to control
pH conditions, Na
+
uptake (
J
Na
in
) remained statistically
unchanged throughout the course of exposure (Figure2A).
Upon initial acid exposure,
J
Na
in
dropped precipitously by
75% within the first hour and remained significantly lower
than pairwise control zebrafish throughout the first 8hours
of exposure (P<.05), but returned to levels not significantly
different from pairwise control zebrafish at 8- 10 hours
(P=.9997) and 10- 12hours (P=.4101).
In addition to
J
Na
in
, we concurrently measured ammonia
excretion (
J
amm
net
) and titratable acidity minus bicarbonate
(
J
TA−HCO
3
−
). These were summed together to yield net acid
excretion
J
H
net
(acid equivalent excretion denoted by negative
values; base equivalent excretion denoted by positive values)
to evaluate potential contributing roles of an NHE- Rh medi-
ated mechanism and/or a VHA- linked ASIC/Na
+
channel
mechanism in the aforementioned restoration of
J
Na
in
during
acid exposure.
J
amm
net
averaged ~840nmolg
−1
hour
−1
and
remained relatively unchanged throughout the time se-
ries in zebrafish held in control pH conditions (Figure 2B;
P>.9514). Compared to pairwise controls,
J
amm
net
in acid-
exposed zebrafish significantly increased only for 0- 1 hours
of exposure (approximately threefold higher, P=.0278) and
returned to control levels throughout the remainder of the
time series. No significant effects of time or treatment were
noted in either
J
TA−HCO
3
−
(Figure2C) or
J
H
net
(Figure2D)
(F
6,68
<2.906, P>.0928), indicating a lack of net acid- base
disturbances at all time periods and treatments.
2.2
|
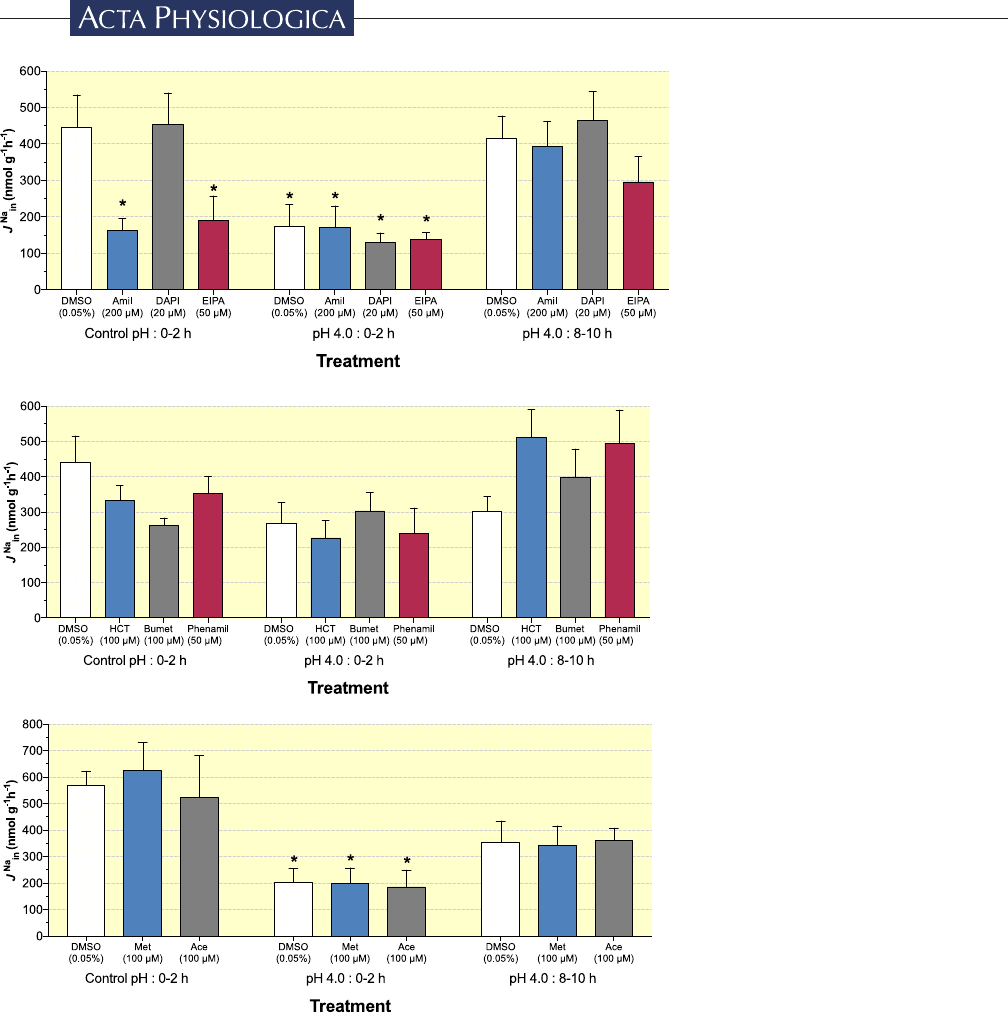
Series 2: Pharmacological
profile of the re- established Na
+
uptake
mechanism during acid exposure
We measured
J
Na
in
in zebrafish (a) during exposure to
control pH water, (b) for 0- 2hours exposure to pH 4.0
(A)
(B)
(C)
(D)

|
5 of 20
CLIFFORD et al.
and (c) for 8- 10hours exposure to pH 4.0. During these
flux treatments, zebrafish were concurrently exposed to
a panel of pharmacological inhibitors (Table1) targeting
key transporters either directly or indirectly involved in
Na
+
uptake (Figure 3). The general trend observed in
vehicle control zebrafish (0.05% DMSO) was a robust
J
Na
in
uptake during control pH conditions, a reduction
in
J
Na
in
during immediate acid exposure [significant in
trial set (a) and (c), with a non- significant reduction in
trial set (b)], and a general return to control rates during
acid exposure after 8 hours pre- exposure. Of all drugs
tested,
J
Na
in
was sensitive only to amiloride and EIPA,
and only during control pH exposure;
J
Na
in
in either
case was inhibited by 60%- 70% compared to vehicle con-
trols. Interestingly, the reductions in
J
Na
in
were com-
parable to those caused by acute exposure (0- 2 hours)
to pH 4.0 (Figure2A), and neither amiloride nor EIPA
caused any further inhibition relative to the respective
vehicle control zebrafish at either 0- 2 or 8- 10hours of
continuing acid exposure. No other differences of note
were observed across all other treatments or drugs (ie
DAPI [Figure2A], phenamil, hydrochlorothiazide and
bumetanide [Figure2B], as well as metolazone and ac-
etazolamide [Figure3C]).
2.3
|
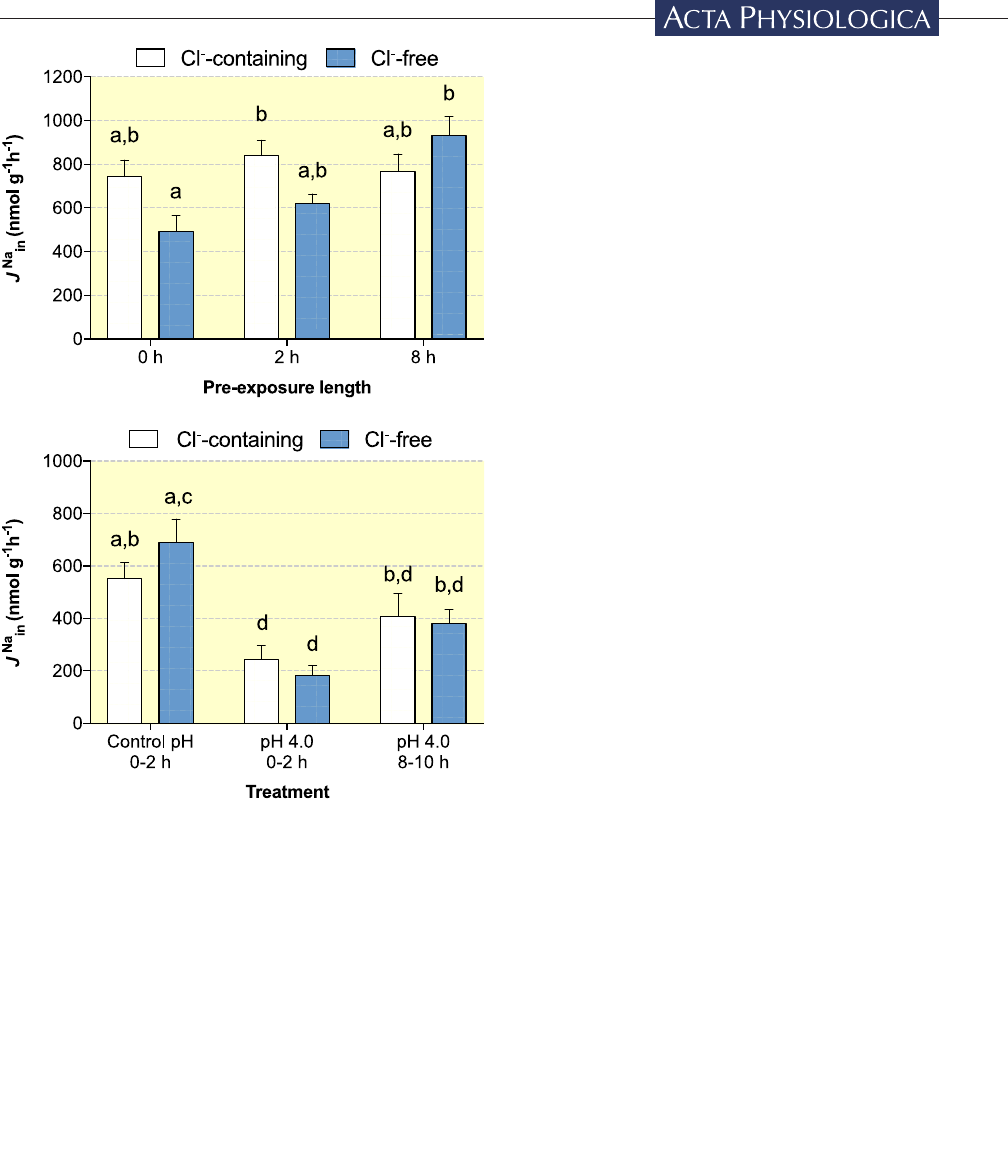
Series 3: Investigating the
role of Cl
in the re- establishment of
J
Na
in
during and after acid exposure
To test for a possible linkage between the restoration of
J
Na
in
and environmental Cl
−
, we characterized
J
Na
in
in two separate exposure/flux protocols, (a) in control
pH water after 0, 2, or 8 hr of pre- exposure to pH 4.0
(Figure4A), and (b) in each of the three treatments de-
scribed in Series 2 (ie control pH and pH 4.0 at 0- 2hours,
and pH 4.0 at 8- 10hours; Figure4B). In both protocols,
J
Na
in
was measured either in Cl
–
- containing or Cl
–
- free
flux media.
In zebrafish transferred from control holding condi-
tions, removal of environmental Cl
–
elicited no significant
differences in
J
Na
in
when characterized in control pH con-
ditions (Figure4A; P=.1813). Furthermore,
J
Na
in
in ze-
brafish pre- exposed to acidic conditions for 2 and 8hours
were not significantly different from 0hour rates in Cl
−
-
containing media (P > .9346), nor were differences in
J
Na
in
detected between the two lengths of acid exposure
(P=.9804). Interestingly, we did note a significant time-
dependent increase in
J
Na
in
in Cl
−
- free trials whereby
8 hours pre- exposed zebrafish exhibited approximately
twofold increase in
J
Na
in
compared to the 0hours control
zebrafish fluxed in the same Cl
−
- free medium (Figure4A;
P=.0023).
When
J
Na
in
was characterized according to the treat-
ments described in Series 2,
J
Na
in
in both Cl
–
- containing
and Cl
–
- free conditions followed the same inhibition and
recovery patterns (Figure4B) seen in Series 1 and Series 2
(ie Figures2A and 3).
J
Na
in
patterns were statistically un-
changed between Cl
–
- containing and Cl
–
- free conditions;
an effect of Cl
–
- free media was not observed (P>.6807).
2.4
|
Series 4: Investigating the
role of environmental [K
+
]
o
in the re-
established Na
+
uptake mechanism during
acid exposure
Zebrafish were exposed to the aforementioned treatments
in either high environmental K
+
(HEK; 50 mM K
+
as
25mM K
2
SO
4
) or in K
+
- free medium (50mM NMDG- Cl
as elevated [Cl
−
] control). Zebrafish in K
+
- free conditions
generally displayed similar pH- dependent inhibition and
time- dependent recovery pattens (Figure 5A) to those
observed in previous experimental series (Figures 2A, 3
and 4B): a significant reduction (~60%) in
J
Na
in
during
initial (0- 2hours) pH 4.0 exposure (P=.0092), followed
by a recovery in
J
Na
in
for 8- 10hours of pH 4.0 exposure
that was not significantly different from
J
Na
in
in control
pH exposed zebrafish (P=.9756). While HEK elicited no
effects on
J
Na
in
during exposure to control pH conditions
(P=.9258), HEK during initial pH 4.0 exposure caused
an even greater inhibition of
J
Na
in
compared to rates
measured during control pH exposure (~95% inhibition;
P<.0001), well below (~85%) the rates observed during
initial pH 4.0 exposure in K
+
- free conditions (P<.0007).
Furthermore, HEK also significantly impacted the recov-
ery of
J
Na
in
following prolonged (8- 10hours) pH 4.0 ex-
posure;
J
Na
in
remained significantly depressed compared
to rates observed in control pH media (~90% reduction,
P<.0001).
The
J
K
net
observed in K
+
- free conditions in control pH
and after immediate exposure to pH 4.0 (0- 2hours) were
FIGURE Time- dependent dynamics of zebrafish ion
regulation during low pH exposure. Groups of zebrafish were
held in either control pH conditions (pH 8.0; white bars) or acidic
water (pH 4.0; blue bars) for up to 12h, and individuals (n=6)
were removed to determine (A) rates of Na
+
uptake (
J
Na
in
) via
22
Na appearance into the animal and (B) net ammonia excretion
(
J
amm
net
) over 1- 2hour periods. Throughout the time series, (C)
J
TA
−
HCO
3
−
(flux of titratable acidity minus
HCO
3
−
; base equivalent
excretion denoted by negative values, acid excretion denoted by
positive values) was also characterized. Respective
J
TA
−
HCO
3
−
values
were added to
J
amm
net
values to calculate (D)
J
H
net
(excretion rates
of net H
+
equivalents). Data are presented as mean±SE. Data not
sharing letters denote significant differences (two- way ANOVA;
Tukey's post hoc test making all comparisons; n=6, P<.05)
6 of 20
|
CLIFFORD et al.
negative and not significantly different from each other
(Figure5B), indicating a small net loss from the animal.
However, zebrafish that had been exposed to pH 4.0 for
8- 10 hours had approximately fourfold increase in out-
wardly directed
J
K
net
. Furthermore, linear regression anal-
ysis of outwardly directed
J
K
net
vs inwardly directed
J
Na
in
in zebrafish exposed to pH 4.0 for 8- 10hours demonstrated
a solid 1:1 correlation [(R
2
=0.9732; slope not significantly
different than 1.0 (F
1,4
=0.5872, P=.4862)] (Figure5C).
This 1:1 relationship was further substantiated in a more
robust linear regression analysis involving all paired
J
K
net
and
J
Na
in
observations from zebrafish which were sub-
ject to prolonged (8- 10hours) pH 4.0 exposure in Series
4 (K
+
- free zebrafish), Series 5 (all zebrafish), and Series
6 (NMDG- and DMSO- control zebrafish) [(R
2
= 0.7073;
slope not significantly different than 1.0 (F
1,44
=0.5042,
P=.4814)] (Figure5D).
J
Na
in
was measured in zebrafish from each of the three
treatments (control pH and pH 4.0 at 0- 2 hours, pH 4.0
at 8- 10hours) in increasing environmental [K
+
]
o
between
38.4µM and 50mM. During control pH exposure, there
was no correlation between
J
Na
in
and environmental
[K
+
]
o
, with a slope that did not differ significantly from 0
(R
2
=0.0132; F
1,40
=1.116, P=.2972) (Figure5E inset). In
contrast,
J
Na
in
measured in both of the pH 4.0 exposures
displayed clear concentration- dependent relationships
with increasing reductions in
J
Na
in
at higher environ-
mental [K
+
]
o
(Figure 5E).
J
Na
in
data measured across
FIGURE Effect of pharmacological
inhibitors on
J
Na
in
in zebrafish during
acid exposure.
J
Na
in
was determined in
control pH (pH 8.0) or pH 4.0 conditions
acutely (0- 2h) or pH 4.0 conditions
following 8hours of acid exposure.
Thirty minutes prior to the addition of
22
Na, zebrafish were first incubated in
flux- media containing (A) Amiloride
(Amil; 200µM), DAPI (20µM) and EIPA
(50µM), (B) Hydrochlorothiazide (HCT;
100µM), Bumetanide (Bumet; 100µM)
and Phenamil (50µM), (C) Metolazone
(Met; 100µM) and Acetazolamide (Ace;
100µM); Vehicle controls (DMSO;
0.05%) were conducted for each drug
panel (white bars). Data are presented as
mean±SE. Data presented with asterisks
(*) denote significant differences from
Control pH:0- 2h/DMSO treatment (two-
way ANOVA; Dunnett's post hoc test
against control groups measured during
control pH conditions in DMSO spiked
flux media; n=6, P<.05)
(A)
(B)
(C)
|
7 of 20
CLIFFORD et al.
increasing environmental [K
+
]
o
were fitted to single-
phase exponential curves and subsequently tested against
one another. This analysis demonstrated that the half-
life constant (interpreted as a proxy to K
i
; the exposure
concentration of K
+
that causes 50% inhibition of
J
Na
in
)
was significantly greater in the prolonged acid exposure
([K
+
]
o
= 1.468 mM) compared to acute acid exposure
([K
+
]
o
=0.5757 mM; F
1,90
=4.999, P=.0278).
2.5
|
Series 5: Profiling the influence of
environmental Na
+
on the dynamics of
J
Na
in
and
J
K
net
during acid exposure
The influence of environmental Na
+
concentration
([Na
+
]
o
) on the apparent Na
+
influx vs K
+
efflux mecha-
nism was evaluated by changing [Na
+
]
o
over a geometric
series during control pH conditions and for 8- 10 hours
of acid exposure. These
J
K
net
and
J
Na
in
data were evalu-
ated against linear and Michaelis- Menten models and the
most appropriate fit was determined for each treatment.
Michaelis- Menten patterns for saturable concentration-
dependence of
J
Na
in
on [Na
+
]
o
were observed both in
zebrafish during control pH conditions and in zebrafish
exposed to pH 4.0 for 8- 10hours (Figure6A). In compar-
ing these patterns, we observed significant differences in
J
max
(453.0±96.3nmolg
−1
hour
−1
in control pH condi-
tions vs 925.8±148.2nmolg
−1
hour
−1
in pH 4.0 condi-
tions) and K
m
(75.8±71.7µM in control pH conditions
vs 391.8±151.4µM in pH 4.0 conditions) (F
2,56
=3.959,
P=.0246).
We also analysed
J
K
net
patterns in the same experimen-
tal series (Figure6B).
J
K
net
in zebrafish tested during con-
trol pH conditions remained stable over all [Na
+
]
o
levels
along a line with a slope that was not significantly different
from zero (R
2
=0.1094; F
1,28
=3.441, P=.0742). However,
zebrafish that had been pre- exposed to pH 4.0 for 8hours
demonstrated a clear [Na
+
]
o
- dependent K
+
efflux pattern
[
J
K
net
(nmol K
+
g
−1
hour
−1
)=302.2±58.65×[Na
+
]
o
mM
+143±36.91; R
2
=0.2505; F
1,27
=26.55. P=.0001].
2.6
|
Series 6: Effect of K
+
transporter
inhibitors on the re- established Na
+
uptake
mechanism during acid exposure
In experimental protocols that mirrored Series 2,
J
Na
in
and
J
K
net
rates were measured in the presence of vari-
ous K
+
channel inhibitors. NMDG control zebrafish and
DMSO control zebrafish displayed similar
J
Na
in
acid-
induced inhibition and recovery patterns as in previous
experiments (Figure7A,C), along with similar stimula-
tion in
J
K
net
efflux following pre- exposure to pH 4.0 for
8 hours (Figure 7B,D). Curiously, in this experimental
series, a non- significant stimulation of
J
K
net
efflux was
also observed in NMDG control zebrafish fluxed imme-
diately in pH 4.0 water, (Figure7B). Ba
2+
did not elicit
any significant changes in either
J
Na
in
or
J
K
net
within
the control pH treatment (Figure7A,B) or during either
acute or prolonged acid exposure in relation to measure-
ments in NMDG- exposed zebrafish during control pH
exposure.
FIGURE Effect of environmental Cl
−
in the re- establishment
of
J
Na
in
during and after acid exposure. Zebrafish were held in
either control pH (pH 8.0) or acidic conditions (pH 4.0) for up
to 8hours prior to the measurement of
J
Na
in
. In (A) all
J
Na
in
measurements were made in control pH conditions.
J
Na
in
was
determined in fish held in either Cl- free (blue bars) or Cl
−
-
containing water (white bars) either before (0hour pre- treatment
control) or immediately after return to control pH conditions after
2 or 8hours of acid exposure. In (B), measurements were either
in Cl- free (blue bars) or Cl
−
- containing water (white bars) at the
indicated pH and time period. Data are presented as mean+SE.
Data not sharing letters denote significant differences (two- way
ANOVA; Tukey's post hoc test making all comparisons; n=6,
P<.05)
(A)
(B)

8 of 20
|
CLIFFORD et al.
FIGURE The influence of environmental [K
+
] on zebrafish
J
Na
in
dynamics during acid exposure. (A)
J
Na
in
was determined in control pH
(pH 8.0) or pH 4.0 conditions acutely (0- 2h) or pH 4.0 conditions following 8- 10h of acid exposure and measurements were carried out in media
that were either high in [K
+
]
o
(HEK, 50mMK
+
, blue bars) or lacking [K
+
]
o
(K
+
- free, 0mMK
+
, replaced with 50mM NMDG, white bars). (B)
net K
+
loss (
J
K
net
) was also measured in all K
+
- free treatments from (A). Unidirectional
J
Na
in
and
J
K
net
observations from zebrafish in prolonged
acid exposure (8- 10h) from (C) the K
+
- free group from the present experimental series and from (D) Series 4 (K
+
- free zebrafish), Series 5 (all
zebrafish) and Series 6 (NMDG- and DMSO- control zebrafish) were regressed and the resulting best fit line tested against a slope of 1 (test details
in figure). (E) unidirectional
J
Na
in
was measured in water with increasing concentrations of [K
+
]
o
in zebrafish during control pH exposure (inset;
black diamonds), acute acid exposure (pH 4.0:0- 2h) exposures; grey squares) or during prolonged acidic conditions (pH 4.0:8- 10h exposure; blue
triangles). Data are presented as mean+SE. Data not sharing letters denote significant differences [(A) two- way ANOVA or (B) one- way ANOVA,
Tukey's post hoc test making all comparisons (n=6; P<.05)]. In (C, D) the dashed line represents y=x, and the solid line represents line of
best fit (95% CI shown as paired dotted lines) with an equation of
J
Na
in
(nmol g
−1
h
−1
)=(1.068±0.089)×
J
K
net
+ (38.58±30.69), R
2
=0.9732,
df=4 in (C) and
J
Na
in
(nmol g
−1
h
−1
)=(1.072±0.106)×
J
K
net
+ (84.81±41.13), R
2
=0.7073, df=43 in (D); the resulting best fit lines were
tested against a slope of 1 (test details in figure). In (E) the dotted line represents the line of best fit as predicted by a linear model with a slope not
significantly different from 0 (inset; R
2
=0.0132; F
1,40
=1.116, P=.2972) and an intercept of 634.1±73.37nmol Na
+
g
−1
h
−1
or a single- phase
exponential decay model (0- 2h:
J
Na
in
(nmol g
−1
h
−1
)=(342.9- 24.38nmolg
- 1
h
−1
)×(e
(−1.204
×
[K+]o mM)
)+24.38; 8- 10h: (382.7- 35.1 nmol g
−1
h
−1
) ×
e
(−0.4723
×
[K+]o mM)
+ 35.1 nmolg
−1
h
−1
). A comparison of fits analysis determined that the half- inhibition concentration in prolonged acid exposure
([K
+
]
o
=1.468mmolK
+
L
−1
) was statistically greater (F
1,90
=4.999; P=.0278) than that in the acute acid ([K
+
]
o
=0.5757mmolK
+
L
−1
) exposure
(A)
(B)
(C)
(D)
(E)

|
9 of 20
CLIFFORD et al.
4- Aminopyridine (4- AP) did not affect
J
Na
in
or
J
K
net
in
any condition (Figure7B,D). Tetraethylammonium (TEA)
also elicited no effects in
J
Na
in
or
J
K
net
during control pH
conditions or for 0- 2hours of pH 4.0 exposure; however,
it did significantly impair the restoration of
J
Na
in
and con-
comitant stimulation of
J
K
net
for 8- 10hours pH 4.0 expo-
sure (Figure7C,D).
2.7
|
Series 7: mRNA expression of
K
+
- dependent Na
+
/Ca
2+
exchangers in
zebrafish gill
Using RT- PCR and Sanger sequencing, we identified
mRNA expression of six genes of the K
+
- dependent Na
+
/
Ca
2+
exchangers (NCKX;slc24) family (slc24a1, slc24a2,
slc24a3, slc24a4a, slc24a5, slc24a6) in zebrafish gill tis-
sue (Figure8; primers and amplicon sizes are shown in
Table2).
3
|
DISCUSSION
Adult zebrafish exhibited marked reductions in Na
+
up-
take at the onset of low pH exposure, which rapidly re-
turned to control rates by 8- 10hours of continued low pH
exposure. Our findings suggest that a novel mechanism
linked to K
+
excretion is responsible for this re- established
J
Na
in
during low pH exposure, which is fundamentally
different from well- established Na
+
uptake mechanisms
in zebrafish. This novel Na
+
uptake mechanism seems to
be electroneutral, relies on outwardly directed 1:1K
+
ef-
flux, is sensitive to TEA but not to inhibitors of the ion-
transporters involved in the reputed mechanisms, and is
fundamentally different from the mechanism that is op-
erational under control pH conditions. Since mammalian
NCKXs (K
+
- dependent Na
+
/Ca
2+
exchangers) match the
kinetics and pharmacology observed in zebrafish exposed
to low pH, and zebrafish gills express mRNA for six nckx
isoforms, these K
+
- dependent Na
+
/Ca
+
exchangers are
primary candidates that could mediate the Na
+
uptake
mechanism described herein.
As expected, zebrafish exhibited an abrupt impairment
(~60%- 75% decrease) in
J
Na
in
in response to acute (2hours)
acid (pH 4.0) exposure (from ~540 nmolg
−1
hour
−1
to ~130
nmol g
−1
hour
−1
; Figure 2A), suggesting inhibition of
the NHE- dominant Na
+
uptake mechanism used during
control conditions. We interpret the remaining
J
Na
in
that
persisted for 0- 2 hours of acid exposure (~130 nmol g
−1
hour
−1
) as non- NHE mediated. It is important to note the
~10000- fold difference in [H
+
]
o
that exists between con-
trol- and acid- exposure conditions, and its direct impact on
J
Na
in
via an NHE. However, during the ensuing time series
at pH 4.0, we found that
J
Na
in
gradually recovered, return-
ing to control rates within ~8- 10hours. To our knowledge,
no other time series data with adult zebrafish during acute
FIGURE Effect of environmental [Na
+
] on the transport
kinetics of
J
Na
in
and
J
K
net
during acid exposure. (A)
J
Na
in
and (B)
J
K
net
were measured in the presence of changing [Na
+
]
o
(75µM-
1.2mM Na
+
) in zebrafish exposed to control pH conditions (pH
8.0; black diamonds) or following 8- 10hours of pre- exposure to
acid conditions (pH 4.0:8- 10h; blue triangles). Data are presented
as mean+SE. Michaelis- Menten models were fitted to
J
Na
in
data, while linear models were fitted to
J
K
net
data. J
max
was
calculated to be 453±96.3nmolg
−1
h
−1
in control pH water and
925.8±148.2nmolg
−1
h
−1
at pH 4.0. K
m
was calculated to be
75.8±71.7µM Na
+
in control pH water vs 391.8±151.4µM Na
+
in
pH 4.0 water. In (B), regression analysis on
J
K
net
supported a linear
model with a slope not significantly different from 0 (R
2
=0.1094;
F
1,28
=3.441, P=.0742) with an intercept of 145.1±17.4nmolK
+
g
−1
h
−1
under control pH conditions and a linear [Na
+
]
o
- dependent
relationship following 8- 10hours of pre- exposure to acid conditions
where
J
K
net
(nmol K
+
g
−1
h
−1
) = 302.2±58.65×[Na
+
]
o
mM
+143±36.91; R
2
=0.4958 (F
1,27
=26.55, P<.0001)
(A)
(B)

10 of 20
|
CLIFFORD et al.
(<12hours) acid exposure have been reported; the clos-
est relevant measurement appears to be 3days post- onset
of acid exposure.
59
These studies reported that adult ze-
brafish exposed to pH 3.8- 4.0 for 3days had similar rates
of Na
+
uptake (measured at low pH) compared to rates
in control zebrafish (measured at circumneutral pH).
After 5days of acid exposure, the kinetic profile of Na
+
uptake with respect to environmental [Na
+
] nearly dou-
bled in J
max
while affinity for Na
+
decreased sixfold (ie K
m
increased).
59
Notably, within 10 hours of acid exposure,
FIGURE Effect of putative K
+
transport inhibitors on unidirectional
J
Na
in
uptake and
J
K
net
in zebrafish during acid exposure.
J
Na
in
(A,
C) and
J
K
net
(B and D) were determined in control pH (pH 8.0) water or during acute (0- 2h) or prolonged (8- 10h) exposure to pH 4.0 water.
Prior to flux measurement, zebrafish were incubated in flux media at indicated pH levels containing either (A, B) Ba
2+
(10 mM; blue bars)
or (C, D) 4- AP (500 µM; blue bars) or TEA (1 mM; grey bars). Vehicle control fluxes were carried out in either (A, B) NMDG (10 MM; black
bars) or (C, D) DMSO (0.05%; white bars). Data are presented as mean ± SE. Data presented with asterisks (*) denote significant differences
from Vehicle control fluxes (two- way ANOVA; Dunnett's post hoc test against (A, B) NMDG or (C, D) DMSO groups measured in control
pH water at 0- 2 hr; n = 6, P < .05)
(A)
(B) (D)
(C)
FIGURE mRNA expression of nckx isoforms in the gills of adult zebrafish. RT- PCR (35 cycles; Phusion polymerase; New England
Biolabs) was conducted on cDNA synthesized from total RNA extracted from gills of control pH (pH 8.0) exposed zebrafish with primers
(Table2) targeting specific isoforms of the slc24 gene family. Amplified products were analysed alongside 1kb ladder (New England Biolabs)

|
11 of 20
CLIFFORD et al.
we too observed a doubling of J
max
and roughly a fivefold
increase in K
m
(Figure6A; discussed below). Whether the
underlying mechanisms responsible for re- established
Na
+
uptake in the current study (within 10 hours) are
the same as those at play following 3- and 5- day exposure
times remains to be investigated.
3.1
|
The case against NHE or the NHE/
Rh metabolon
The recovery of Na
+
influx to control rates during con-
tinued acid exposure was insensitive to both amiloride
(inhibitor of NHEs, Na
+
channels, and ASICs
34,60
) and
EIPA (NHE inhibitor
34
) (Figure 3A). Rescue of NHE
function by an Rh- metabolon during acid exposure
would involve sustained elevations in
J
amm
net
; however,
we only observed a transient increase in
J
amm
net
that was
limited to the earliest time point (0- 1hour) (Figure2B).
The transient rise in
J
amm
net
may be explained by imme-
diate exposure to low pH creating an acidic
NH
4
+
- sink
(acid- trapping) for metabolically derived NH
3,
sud-
denly stripping the organism of NH
3
before returning
to control flux rates fuelled by metabolism.
61
Overall,
the inhibitor results, lack of a persistent increase in
J
amm
net
, throughout exposure and thermodynamic chal-
lenges previously described effectively eliminate a role
for NHEs – alone or as part of an Rh- mediated metabo-
lon – in the re- established Na
+
uptake during acid ex-
posure. In fact, given the thermodynamic constraints
for NHE, we might predict a down- regulation of apical
NHE expression within the gill ionocytes so as to pre-
vent a reversal of Na
+
/H
+
exchange that would further
exacerbate Na
+
loss.
3.2
|
The case against Na
+
channels/
ASICs
Na
+
movement through Na
+
channels/ASICs is electro-
genically tied to VHA- mediated H
+
excretion, and car-
bonic anhydrase (CA) activity is predicted to provide H
+
as substrate for VHA. Thus, a Na
+
channel/ASIC mecha-
nism would entail an increase in net acid efflux. However,
we noted no overall effects of time or treatment in either
J
TA−HCO
3
−
(Figure2C) or
J
H
net
(Figure2D). Taken together
with the lack of sensitivity to DAPI (Figure3A; ASIC in-
hibitor
36
), phenamil (Figure3B; Na
+
channel inhibitor
39
)
and acetazolamide (Figure 3C; CA inhibitor
10,47
) during
either acute (0- 2hours) or prolonged (8- 10hours) acid ex-
posure, these results indicate that the re- established
J
Na
in
during acid exposure was not mediated via ASIC or Na
+
channels.
While insensitivity to phenamil was expected given
the lack of an identifiable ENaC orthologue in zebraf-
ish genome databases
62
(also undetected within current
GRCz11 assembly, GCA_000002035.4), insensitivity to
DAPI during control conditions was surprising given that
zebrafish gills express mRNA for all six zebrafish ASIC
isoforms over a wide range of environmental [Na
+
] (~50 to
1300µM).
9
Furthermore, Dymowska et al
9
reported that
roughly 50% of Na
+
uptake in adult zebrafish acclimated
to low environmental ion levels and control pH ([Na
+
]:
~500µM, [Cl
–
]: ~300µM, [Ca
2+
]: ~1.2mM, pH ~8.5) was
sensitive to DAPI (10 µM) and amiloride (200 µM), but
not EIPA (100µM).
9
However, in that same study, zebraf-
ish exposed to ultra- low environmental ion levels and
slightly acidic pH ([Na
+
]: ~50µM, [Cl
–
]: ~60µM, [Ca
2+
]:
~300µM, pH ~6) exhibited no sensitivity whatsoever to
either DAPI or EIPA. Both the ultra- low water chemistry
TABLE Transcript- specific primers used for RT- PCR
Transcript Accession number Primer sequence (5′- 3′)
Annealing
temperature, °C
Amplicon
(bp)
slc24a1 XM_021473276.1 F: CAT ACC CCT GCA TCT TTT AGC G 61 2411
R: ACC TGT GAA AGA ACT GTG ATG TC
slc24a2 XM_017355745.2 F: CCG TAA GTC TGT GGG ATT CTT 61 2361
R: TGG ATG TCC TTG CCT CAT TAA A
slc24a3 XM_680210.8 F: GAA CTG GCA CCA AAC TGA CG 61 2268
R: GAA GGA GAG CCT TTC TGC GT
slc24a4a XM_009293194.3 F: CCG ATC CCG AGC CTG ATT TT 61 1960
R: TGG TTC AAA GCC CAT GGA GAA
slc24a5 NM_001030280.1 F: TGT GTG TGT TCT CCG TCA TC 62 1719
R: CGC ACT TTG ACT TCT CTT GTA TTT
slc24a6 XM_021474309.1 F: TGG AAA GGG CAC ATA TCG GTA A 64 2153
R: AAT AAG GCA GTG ACT GGG GG

12 of 20
|
CLIFFORD et al.
used by Dymowska et al
9
and the low pH conditions in
the present study would present adverse gradients for the
function of an NHE for Na
+
uptake. Since both studies
reported a similar lack of pharmacological blockade with
either amiloride, EIPA, DAPI or phenamil, the putative
H
+
- linked Na
+
uptake models do not seem to be func-
tional under these conditions. A possible explanation may
be that ASICs can function only when fish are exposed to
moderately low [Na
+
]
o
and pH but not in either ultra- low
[Na
+
]
o
or very low pH.
3.3
|
The case against NCC
To evaluate the putative role for NCC in the recovery
of
J
Na
in
during acid exposure, we tested a possible link
to environmental Cl
–
. One flux experiment utilized
Cl
–
- free media to evaluate the role of NCC following
transfer from acid exposure to control pH conditions
(ie recovery from an acid exposure), while a separate
flux experiment evaluated the role of NCC during the
acid exposure. While Kwong and Perry
13
noted stimu-
lations in
J
Na
in
following transfer to control pH condi-
tions in larval zebrafish, we observed no such effect in
our adult zebrafish (Figure4A), perhaps indicating life
stage- specific differences. In addition, removal of en-
vironmental Cl
−
did not affect the ability of our adult
zebrafish to recover
J
Na
in
following low pH- exposure
at any time- point, nor did it inhibit the residual pH-
independent
J
Na
in
observed during acute low pH expo-
sure (Figure4B). Furthermore, applications of HCT and
metolazone (NCC inhibitors
37,43– 46
), or bumetanide (an
inhibitor of both NCCs and NKCCs
37,42
), also had no ef-
fects on Na
+
uptake in any flux treatment (Figure3B,C).
Most importantly, the recovery of
J
Na
in
at 8- 10 hours of
continued acid exposure was not attenuated in Cl
–
- free
conditions. From these results, combined with the ther-
modynamic challenges raised in the Introduction, we
can conclude that NCC is not a relevant mechanism ex-
plaining the return of Na
+
uptake during acid exposure.
3.4
|
The case for a K
+
- dependent Na
+
uptake mechanism
After systematically ruling out roles of each of the three
putative Na
+
uptake mechanisms in the re- established
J
Na
in
during acid exposure, we re- visited first principles
of ion exchange in relation to water chemistry to assess
what other possible driving gradients could be used to re-
establish
J
Na
in
in low pH conditions. While environmental
[K
+
]
o
in our experiments was extremely low (~4µM), K
+
is
the primary inorganic ion in the intracellular pool
63
with
an estimated average intracellular [K
+
] ([K
+
]
i
) in teleost
gill ranging from ~14- 90 mM.
64,65
Furthermore, Na
+
- K-
ATPase activity in ionocytes is bound to result in [K
+
]
i
in
the upper range (or perhaps higher) along with very low
[Na
+
]
i
in these cells. The resulting diffusion gradient (4µM
[K
+
]
o
vs 14000- 90000µM [K
+
]
i
) could provide a very large,
outwardly directed ion- motive force. And while K
+
extru-
sion in exchange for Na
+
uptake has been traditionally
argued against because of the low K
+
permeability of gold-
fish (Carassius auratus) gills,
66
to our knowledge there are
no studies examining K
+
efflux rate in conjunction with
unidirectional Na
+
uptake during low pH exposure. That
said, a limited number of studies examining net Na
+
and
K
+
efflux in several species of Amazonian fishes have re-
ported stimulations in
J
K
net
either within 1hour of low pH
exposure (pH
≤
3.5)
67
or following gradual decrements in
water pH.
68
Intriguingly, in the latter study, stimulations of
J
K
net
loss following 18hours of low pH (pH 4.0) exposure
were associated with reductions in
J
Na
net
loss, compared
to measurements at 1 hour of exposure in all three fish
species studies [tamoatá (Hoplosternum littorale), matrin-
cha (Brycon erythopterum) and tambaqui (Colossoma ma-
cropomum)]; however unidirectional Na
+
fluxes would be
needed to correctly compare these results to our own.
If we apply the intracellular [K
+
]
i
and environmental
[K
+
]
o
to models of electroneutral counter- transport,
20
we
find that K
+
efflux could clearly drive electroneutral Na
+
/
K
+
exchange. Therefore, we tested whether K
+
efflux was
responsible for re- establishing Na
+
uptake during low
pH exposure by measuring
J
Na
in
in HEK (50mMK
+
). By
eliminating (or perhaps reversing) K
+
efflux, HEK would
be predicted to inhibit K
+
- dependent Na
+
uptake but only
during acid exposure (Figure5A). Indeed, HEK had no ef-
fect on
J
Na
in
during control pH exposure, which matched
the observed low K
+
permeability in goldfish gills,
66
but
remarkably, HEK induced a near- complete abolishment
of
J
Na
in
during both short- term (0- 2 hours) and contin-
ued (8- 10 hours) acid exposure. Thus, disruption of the
outwardly directed K
+
gradient effectively abolished the
NHE- independent mediated
J
Na
in
that persisted during
low pH exposure. These results support a K
+
- efflux- driven
Na
+
uptake mechanism that gets activated and progres-
sively gains importance during exposure to low environ-
mental pH.
For completeness, we also tested the effect of K
+
- free
water on
J
Na
in
but found no effects during control condi-
tions, during short- term (0- 2hours) acid exposure to low
pH (ie zebrafish experienced the typical ~60% reduction in
J
Na
in
) or during continued (8- 10hours) acid exposure (ie
zebrafish fully recovered
J
Na
in
) (Figure5A).
We next examined the net K
+
loss (
J
K
net
) during acid
exposure. In zebrafish exposed to K
+
- free conditions,
J
K
net
was negative (ie a small net loss from the animal) with

|
13 of 20
CLIFFORD et al.
similar rates during control pH conditions and during
acute (0- 2hours) pH 4.0 exposure (Figure5B). However,
zebrafish continuously exposed to pH 4.0 for 8- 10hours
experienced an approximately fourfold increase in out-
wardly directed
J
K
net
. This increase, paired with the strong
1:1 relationship between K
+
loss and Na
+
uptake rates ob-
served in Series 4 (Figure5C) and further supported by
regression of all 8- 10hours
J
K
net
and
J
Na
in
data collected
from Series 4 (K
+
- free zebrafish), Series 5 (all zebrafish)
and Series 6 (NMDG- and DMSO- control zebrafish)
(Figure5D) indicated a functional relationship between
the two, but only during low pH conditions.
Importantly,
J
Na
in
was independent from environmen-
tal [K
+
]
o
during control conditions but was strongly inhib-
ited by increasing [K]
o
during both acute and sustained acid
exposure (Figure5E), supporting the idea that K
+
efflux
plays a critical role in re- establishing Na
+
uptake during
acid exposure. Furthermore, our kinetic analysis revealed
that the half- life constant (interpreted as a proxy to K
i
; the
exposure concentration of K
+
that causes 50% inhibition
of
J
Na
in
) was significantly greater following prolonged
acid exposure compared to acute acid exposure. Thus, the
potency of environmental [K
+
]
o
as a competitive inhibitor
diminished following 8- 10hours of exposure, suggesting
a progressive upregulation of the mechanism responsible
for the increased
J
Na
in
. Put another way, during continued
acid exposure, zebrafish are progressively upregulating
an Na
+
/K
+
exchange mechanism which in effect elicits a
higher internal affinity for K
+
.
We also found that prolonged acid exposure caused
dramatic shifts in the [Na
+
]
o
- dependent kinetics of both
J
Na
in
and
J
K
net
. With regards to
J
Na
in
we found that J
max
roughly doubled in response to 8- 10hours of acid expo-
sure, while the K
m
was approximately fivefold greater
(Figure6A). Thus, maximum Na
+
transport capacity dou-
bled, whereas Na
+
transport affinity decreased by fivefold
after 8- 10hours exposure to pH 4.0. In examining
J
K
net
patterns in the same experimental series,
J
K
net
was deter-
mined to be independent of [Na
+
]
o
during control pH con-
ditions, while 8- 10hours of acid exposure induced a
J
K
net
pattern that was strongly dependent upon [Na
+
]
o
suggest-
ing a clear linkage between K
+
efflux and Na
+
uptake in
longer- term acid- exposed zebrafish (Figure6B). Taken to-
gether, these data indicate the upregulation of a novel Na
+
uptake mechanism during acid exposure with markedly
different kinetics, substrates and ion- motive forces com-
pared to the NHE- dependent mechanism utilized during
control conditions.
In vertebrates, K
+
is a major intracellular monovalent
cation and is maintained at >20- fold higher than extracel-
lular K
+
levels
69
and up to ~22500- fold higher than [K
+
]
o
observed in the current study. K
+
is generally available
via the diet in excess of requirements.
70
Plasma [K
+
] for
freshwater fishes ranges from 4 to 5mM
71
while average
intracellular [K
+
] throughout the body ranges 80- 90mM.
Assuming a blood volume of ~4% and a ~66% intracel-
lular volume in a 500- mg zebrafish, the total estimated
on- board K
+
would be ~30 000 nmols K
+
, which could
sustain the upregulated K
+
- dependent
J
Na
in
operating at
~400nmolg
−1
hour
−1
for ~15hours before experiencing
a 10% reduction in whole- body K
+
(hypokalaemia). These
calculations illustrate that a putative Na
+
/K
+
exchange
mechanism could sustainably operate during acid expo-
sure indefinitely, so long as the animal can replenish K
+
stores by feeding.
3.5
|
Evaluating potential K
+
transport pathways
K
+
is transported across membranes via a variety of trans-
port proteins including NKA, H
+
- K
+
- ATPase (HKA),
NKCC, and NCKXs. For NKA to play a direct role, the
transporter would need to be operating on the apical
surface of gill ionocytes and in the reverse direction. To
our knowledge, there are no reports about apical NKA
in gill cells, operating in either direction. Similarly, HKA
takes up, rather than excretes, K
+
; in any case, the cur-
rent zebrafish GRCz11 genome assembly does not possess
HKA homologues. Furthermore, a mechanism involving
HKA would rely on the concomitant involvement of a
Na
+
channel as well as CA, for which we found no evi-
dence (Figure3A- C). A lack of inhibition by bumetanide
on the restored
J
Na
in
(Figure3B) rules out NKCC as well.
K
+
channels are subcategorized into Ca
2+
- activated, tan-
dem pore domain, inward rectifying, and voltage- gated
K
+
channels. Recent studies have implicated the apical
inwardly rectifying K
+
channel, ROMK (also known as
kcnj1 or kir1.1) in K
+
secretion in freshwater gill iono-
cytes. However, if K
+
channels were indeed playing a role,
it would again likely involve linkage to a Na
+
channel
mechanism.
Ba
2+
is a broad K
+
channel inhibitor that targets Ca
2+
-
activated K
+
channels, tandem pore K
+
channels, along with
ROMK and other inwardly rectifying K
+
currents.
16,48– 50,72,73
We observed no inhibitory effect of Ba
2+
on
J
Na
in
or
J
K
net
during control pH conditions or during either acute or
prolonged acid exposure in relation to measurements in
NMDG- exposed zebrafish during control pH exposure. 4- AP
(inhibitor of voltage- gated K
+
channels
74
) did not elicit any
deviations from the typical
J
Na
in
inhibition and recovery
patterns in any of the treatments (Figure7C,D). TEA (a non-
specific inhibitor of Ca
2+
- activated K
+
channels,
53,54
voltage-
gated K
+
channels,
55
NKA
56
and NCKXs
57,58
) also elicited no
effects on either
J
Na
in
or outward
J
K
net
during either control
pH or acute pH 4.0 conditions. Intriguingly, TEA did inhibit

14 of 20
|
CLIFFORD et al.
both the restoration of
J
Na
in
and concomitant increase in
outward
J
K
net
in zebrafish during prolonged acid exposure.
Since the Ba
2+
and 4- AP results had ruled out roles for Ca
2+
-
activated K
+
channels or Kv1 channels, and the lack of effect
of TEA on
J
Na
in
during control pH exposure rules out NKA,
we are left with the possibilities that either NKCXs play a
role in the K
+
- dependent
J
Na
in
mechanism that is activated
upon acid exposure, or that we have discovered a completely
new mechanism.
NCKXs are a family of low- affinity/high capacity ion
transporters which exchange inward- moving Na
+
for
outward- moving K
+
and Ca
2+
.
75
Mammals possess five
NCKX genes (NCKX1- 5) that are often regarded as Ca
2+
transporters with putative roles in sperm flagellar beating,
76
retinal cone phototransduction,
77
skin pigmentation
78
and
neuronal function.
79
In addition, NCKXs are expressed in
vascular smooth muscle, thymus, lungs, epidermal cells,
intestine and kidney
80– 83
; however, their roles in transep-
ithelial Na
+
transport has never before been considered.
Zebrafish possess seven nckx genes within their annotated
genome; of these, we were able to detect mRNA expression
of six (slc24a1, slc24a2, slc24a3, slc24a4a, slc24a5, slc24a6)
within gill tissue through RT- PCR (Figure8). The proposed
stoichiometry of NKCX1 and NCKX2 has been determined
experimentally as 4Na
+
/1Ca
2+
+1K
+84
; however, these rela-
tionships have yet to be elucidated for other isoforms and
in other species. Given that the NCKX family mediates K
+
-
dependent Na
+
transport, these transporters currently are
the most likely molecular candidates to consider for the ob-
served re- established Na
+
uptake.
3.6
|
Summary and significance
During control conditions,
J
Na
in
uptake in adult zebrafish
primarily occurs via well- characterized NHE- dependent
mechanisms. However, when zebrafish are exposed to
low pH water, NHE function is thermodynamically inhib-
ited, yet
J
Na
in
is gradually restored back to control rates
over time. Pharmacological inhibitor experiments using
concentrations known to be effective in previous studies
in teleosts (Table1) failed to attribute this restored Na
+
uptake to reputed models.
To overcome the limitations often cited in inhibitor-
based studies, we made use of alternative approaches to
further evaluate potential contributions from established
models in the restored
J
Na
in
namely, the NHE- Rh metab-
olon model was evaluated by measuring
J
amm
net
and
J
H
net
measurements; the VHA- linked ASIC/Na
+
channel was
evaluated by measuring
J
H
net
and the NCC model was
evaluated by measuring
J
Na
in
in Cl
–
- free media.
Thus, by considering our inhibitor data alongside
these alternative approaches, we were able to rule out
the involvement of existing Na
+
uptake models in fish.
Instead, through consideration of first principles of ion-
exchange, we identified and functionally characterized a
novel Na
+
uptake mechanism that relies on the equimolar
efflux of K
+
in adult zebrafish. The presence of six nckx
isoforms in zebrafish gills combined with the observed
sensitivity of the K
+
- dependent Na
+
uptake to TEA inhibi-
tion points to NCKXs as likely molecular candidates medi-
ating this novel mechanism; however, this will need to be
confirmed through future molecular, cell biology, kinetics,
and histochemical experiments.
It is important to note that the zebrafish has now be-
come a model system for understanding ion transport at
low pH
9,13,19,28– 30,32,33,59
as discussed in detail by Kwong
et al.
85
We now know that many other teleosts of the Order
Cypriniformes (to which zebrafish belong), as well as the
Orders Perciformes, Characiformes, Siluriformes and
Cichliformes, also inhabit waters at pH 4.0 and below, yet still
maintain Na
+
homeostasis.
85– 87
Given the wide geographic
distributions and phylogenetic relationships in these teleost
species, it would be intriguing to determine if the ability
to invoke similar K
+
- dependent Na
+
uptake mechanisms
allow these fishes to inhabit low pH environments, provid-
ing a competitive advantage and thus allowing for their ex-
pansion to their realized niches. Our findings thus provide
an impetus to look for similar functions in fish inhabiting or
transiting low pH environments such as Amazonian water
bodies and acid rain contaminated lakes.
86,87
In summary, the functional identification of this novel
Na
+
uptake pathway opens a new avenue within the study
of Na
+
uptake in freshwater fishes and more broadly the
fields of ion and acid- base regulation and comparative
physiology. Future elucidation of the molecular mecha-
nism responsible for Na
+
/K
+
exchange is a crucial next
step, as is understanding how the mechanism is regulated,
and specifically identifying its cellular location. Zebrafish
have at least five different types of gill ionocytes.
88
Does
this new mechanism reside within one or more types of
these characterized ionocytes, or are there other subtypes
that are yet to be identified? Are there other environmental
challenges where this mechanism plays a role for teleosts?
Is there some inherent cost of K
+
- dependent Na
+
uptake
which makes it only worth employing during low pH expo-
sure? These and many other questions regarding this novel
K
+
- dependent Na
+
uptake mechanism await investigation.
4
|
MATERIALS AND METHODS
4.1
|
Experimental animals and holding
Zebrafish (Danio rerio; 150- 500mg; total N=701) were
obtained from a local pet store and were kept in two

|
15 of 20
CLIFFORD et al.
50- L aerated glass aquaria (up to 200 fish per tank), with
a 14:10 hours light/dark photoperiod at room tempera-
ture (20- 22°C). Upon acquisition, fish were acclimated
for at least 2weeks to holding conditions (Na
+
: 1.1mM,
Ca
2+
: 2.1mM, Cl
–
: 4.1mM, Mg
2+
: 6.5µM, K
+
: 3.84µM,
SO
4
2−
: 10.41µM, pH ~8.0) prior to any experimentation.
Tanks were supplied with gentle aeration and were fit-
ted with a biological filter. Water was refreshed bi- weekly
with a 50% water change with prepared holding water.
Fish were fed commercial fish food (Tetramin
®
tropical
flakes, Tetra Spectrum Brands Pet LLC), ad libitum over
30minutes, three times a week, with food being withheld
for 48 hours prior to experimentation. Fish were trans-
ferred from general holding to exposure aquaria (15- L
aquaria with aeration) to settle overnight prior to experi-
mentation. All zebrafish were used under the University
of British Columbia Animal Care Protocol A14- 0251.
4.2
|
Reagents
Unless noted otherwise, all chemical compounds, rea-
gents and enzymes were supplied by Sigma– Aldrich
Chemical Company. Ethyl 3- aminobenzoate meth-
anesulfonate (MS222) was obtained from Syndel labo-
ratories (Nanaimo, BC, Canada). Radio- labelled
22
Na
(as
22
NaCl) was purchased from Perkin Elmer, activ-
ity=1µCi µL
−1
). All reagents and buffers were prepared
in deionized water and all pharmacological agents were
dissolved in 0.05% DMSO, unless otherwise specified.
Vehicle control experiments with 0.05% DMSO alone
were also performed.
4.3
|
Experimental protocols
4.3.1
|
Series 1: Time- course
dynamics of zebrafish ion- regulatory status
during acid exposure
Preliminary rangefinder experiments indicated that acute
(2- hour) pH 4.0 exposure elicited a ~65% inhibition in
J
Na
in
compared to rates observed in control pH exposed
zebrafish, while animals exposed to pH 3.5 exhibited a
~90% inhibition. While no deaths were observed at either
of the low pH exposures, towards the end of the 2hour
pH 3.5 exposure, zebrafish appeared inactive and listless;
thus, we elected to utilize pH 4.0 as an exposure pH for the
remainder of our experiments.
Zebrafish (n = 42 per group) were exposed to either
control (pH 8.0 ± 0.1) or acidic (pH 4.0 ± 0.05) water
for up to 12hours. To maintain acidic conditions during
exposure, a Radiometer (Radiometer- Copenhagen,
Brønshøj, Denmark) pH- stat system consisting of a pH
meter (PHM82), combination glass- bodied pH electrode
(GK24O1C) and an auto- titration controller (TTT- 80) me-
tered the addition of acid titrant (0.1M HCl) via a solenoid
valve into the experimental chamber. At marked times
(0,1, 2, 4, 6, 8 and 10hours) during the 12- hour exposure
period, subsets of individual zebrafish (n=6) from each
treatment were transferred from exposure aquaria into
individual 50- mL flux chambers (one fish per flux cham-
ber) containing known volumes of pH- matched media (ie
either pH 8.0 or 4.0) spiked with
22
Na (0.02 µCi mL
−1
);
aeration was provided to promote mixing. Rates of uni-
directional Na
+
uptake (
J
Na
in
) were determined using
standard radiotracer methods, measuring the appear-
ance of
22
Na in the fish over a 1- 2hours period. During
flux experiments, water samples (15- mL) were removed
both immediately following the addition of fish and at
the conclusion of the flux period for later determination
of
22
Na gamma radioactivity, total [Na
+
], total ammonia
([
NH
4
+
] + [NH
3
]) and titratable acidity minus bicarbonate
(TA- HCO
3
-
). Following final water sample collection, ze-
brafish were quickly washed in a high salt bath (200mM
NaCl of appropriate pH) for 1minute to rinse residual ra-
dioactivity from the cutaneous surface, then euthanized
via overdose of MS222 (1g L
−1
MS222 buffered with 2g
L
−1
NaHCO
3
) then individually weighed and analysed for
22
Na gamma radioactivity.
4.3.2
|
Series 2: Pharmacological
profile of the re- established Na
+
uptake
mechanism during acid exposure
Zebrafish (n=6 per treatment) were transferred directly
from acclimation/exposure conditions to flux chambers
containing media spiked with DMSO (0.05%; vehicle
control) or one of several pharmacological inhibitors tar-
geting various Na
+
and other related acid/base transport
mechanisms (See Table1 for inhibitors, putative targets,
exposure concentrations and references to previous stud-
ies substantiating these concentrations). Zebrafish held
in non- acidic conditions were transferred to individual
chambers held at either control (8.0) or acidic (4.0) pH
levels, while zebrafish exposed to pH 4.0 for 8hours (as
above) were transferred to individual chambers held con-
tinuously at acidic pH 4.0. For these flux protocols, ze-
brafish were allowed to incubate in inhibitor- spiked flux
media for 30minutes to allow time for the blocker to take
effect. Flux chambers were then inoculated with
22
Na
(0.02µCimL
−1
), gently pipette- mixed, then after 5min-
utes, sampled for water (15mL) to initiate the beginning of
a 1.5- hours flux period. Flux protocols otherwise matched
those that were adhered to in Series 1 experiments.

16 of 20
|
CLIFFORD et al.
4.3.3
|
Series 3: Investigating the role of [Cl
−
]
in the re- establishment of
J
Na
in
during and
after acid exposure
To test for the influence of environmental [Cl
–
] on Na
+
uptake, zebrafish were exposed to either control or acidic
conditions for up to 8hours (as above). Following either
0hour (no- exposure control), 2, or 8hours of acid expo-
sure, a subset of zebrafish (n=6 per treatment) was trans-
ferred into individual flux chambers filled with either
Cl
–
- containing media (2.032mM CaCl
2
; 1.1mM NaHCO
3
;
6.5 µM MgSO
4
; 3.91 µM CaSO4; 3.84 µM KCl) or Cl
–
-
free media (2.036mM CaSO
4
; 1.1mM NaHCO
3
; 6.5µM
MgSO
4
; 1.92µM K
2
SO
4
), both of which were set to control
pH and spiked with
22
Na (0.02µCimL
−1
). A second sub-
set of zebrafish (n=9 per group) undergoing exposure to
control or acidic conditions was similarly transferred to
22
Na
+
- spiked media that were either Cl
–
- containing or Cl
–
- free, however, in this iteration the flux media was set to
either control pH, or pH 4.0 by titration with 0.1M H
2
SO
4
,
so as to match the pH condition from which the zebrafish
had been transferred. Flux protocols (2hours) were oth-
erwise carried out as in described in Series 1, with water
samples (15mL) measured for total [Na
+
] and both water
samples and euthanized fish analysed for
22
Na gamma
radioactivity.
4.3.4
|
Series 4: Investigating the role of
environmental [K
+
]
o
in the re- established Na
+
uptake mechanism during acid exposure
Pre- flux exposure conditions and post- transfer flux
treatments matched those protocols used in Series 2 (ie
fluxes measured at control pH, and at pH 4.0 at 0- 2 and
at 8- 10hours after transfer to pH 4.0; n=6 per group).
However, in this series of experiments, a subset of ze-
brafish was transferred into
22
Na- spiked (0.02µCimL
−1
)
flux media modified to be either nominally K
+
- free
or high in [K
+
]
o
. The composition of the K
+
- free me-
dium was 3.84µM KCl, 50mM N- Methyl- D- glucamine
(NMDG) 2mM CaCl
2
, 1mM NaHCO
3
, 6.5µM MgSO
4
,
3.91 µM MgSO
4
, and the high [K
+
]
o
medium (HEK)
was 25 mM K
2
SO
4
, 3.84 µM KCl, 2 mM CaCl
2
, 1 mM
NaHCO
3
, 6.5µM MgSO
4
, 3.91µM MgSO
4
. Both experi-
mental media were first titrated to pH 8.0 with H
2
SO
4
,
and the low pH medium was thereafter titrated to pH 4.0
with HCl. A second subset of zebrafish (n=6 per group)
was transferred to and similarly tested in media contain-
ing different [K
+
]
o
(0.5, 1, 2.5, 5, 10, 25mM; prepared by
mixing aforementioned K
+
- free and HEK media in ap-
propriate proportions) set to the above pH conditions.
Flux periods (2 hours) were initiated upon removal of
the initial water sample (15mL) and otherwise matched
protocols were adhered to in Series 2. In addition to the
measurement of total [Na
+
]
o
and radioactive
22
Na, water
samples were also measured for [K
+
]
o
(see Water analy-
sis below).
4.3.5
|
Series 5:
Profiling the influence of environmental Na
+
on the dynamics of
J
Na
in
and
J
K
net
during
acid exposure
In this experimental series, the influence of [Na
+
]
o
on
J
Na
in
and
J
K
net
) during control conditions and following
8hours of acid exposure (pH 4.0:8- 10hours) was investi-
gated by transferring acclimated/exposed zebrafish (n=6
per group) to
22
Na- spiked flux chambers containing differ-
ent [Na
+
]
o
(75, 150, 300, 600, 1200µM) prepared by mix-
ing volumes of Na
+
- containing (2mM Na- HEPES, 2mM
CaCl
2
, 6.5µM MgSO
4
, 3.91µM CaSO
4
, 3.84µM KCl) and
Na
+
- free [2mM NMDG (NMDG- SO
4
to pH 8.0 and there-
after NDMG- HCl to pH 4.0, 2mM CaCl
2
, 6.5µM MgSO
4
,
3.91 µM CaSO
4
, 3.84µM KCl)]. Prior to the addition of
zebrafish, flux media were spiked with
22
Na (ranging from
12.5- 20 nCi mL
−1
) such that the final specific activity with
respect to Na
+
content was 16.67- 33.33µCimmol
−1
in the
bathing solution. Flux protocols and sampling otherwise
matched those described in Series 2.
4.3.6
|
Series 6: Effect of K
+
transporter
inhibition on the re- established Na
+
uptake
mechanism during acid exposure
Pre- flux exposure conditions and post- transfer flux treat-
ments matched those protocols used in Series 2. Zebrafish
(n = 6 per group) were transferred to flux chambers
containing putative inhibitors and chemical antagonists
against known K
+
transport pathways (See Table 1). A
subset of zebrafish was transferred to either barium- spiked
flux media (10mM BaCl
2
, 10mM mannitol, 2mM CaCl
2
,
1mM
NaHCO
3
, 6.5µM MgSO
4
, 3.91µM CaSO
4
, 3.84µM
KCl) or NMDG- spiked flux media (20 mM NMDG- SO
4
,
2 mM CaCl
2
, 1 mM
NaHCO
3
, 6.5 µM MgSO
4
, 3.91 µM
CaSO
4
, 3.84µM KCl) as a control, while a second subset
of zebrafish (n =6 per group) was transferred to media
spiked either with DMSO (0.05%) or the pharmaceutical
inhibitor dissolved in DMSO). The pH of these flux solu-
tions was set to pH 8.0 with H
2
SO
4
and thereafter to pH
4.0 with HCl. Fish were allowed to incubate for 30minute
prior to the addition of
22
Na (0.02µCimL
−1
), after which
flux protocols (1.5hours) were carried out as described in
Series 2.

|
17 of 20
CLIFFORD et al.
4.3.7
|
Series 7: mRNA
expression of K
+
- dependent Na
+
/Ca
2+
exchangers in zebrafish gill
Lab- acclimated zebrafish were euthanized, and gill tissue
was excised and snap- frozen in RNA later. Total RNA was
isolated from tissue using a commercially available kit
(RNeasy
®
Mini Kit; Qiagen) according to the manufactur-
er's protocol and quantified using a NanoDrop
®
ND- 1000
UV- vis spectrophotometer (NanoDrop Technologies).
First- strand cDNA synthesis was conducted from 1µg of
RNA with random hexamer primers using a commercially
available kit (Superscript™ IV First- Strand Synthesis
System; Invitrogen) per manufacturer's instructions.
RT- PCR primers targeting zebrafish- specific mRNA
transcripts of nckx isoforms (slc24a1, slc24a2, slc24a3,
slc24a4a, slc24a5, slc24a6) were designed using NCBI-
Primer- BLAST (Table 2). Amplification was performed
using Phusion polymerase (New England Biolabs) and the
following reaction conditions; 98°C for 1 minute of ini-
tial denaturation followed by 35 cycles of denaturation at
98°C for 10seconds, annealing at 61- 64°C for 30seconds
(Table2), and elongation at 72°C for 1minute 45seconds,
followed by a final elongation at 72°C for 10minutes. PCR
products were visualized by 1% agarose gel electrophore-
sis followed by SYBR™Safe staining (Invitrogen). Bands
of interest were excised and purified; sequence identity of
amplified products was confirmed by Sanger sequencing
(Retrogen, Inc.).
4.4
|
Water analysis
Water samples were analysed for
22
Na gamma radioactiv-
ity and total [Na
+
]
o
in all experimental series, and addi-
tionally for total [ammonia] (T
[Amm]
), [K
+
]
o
, and titratable
acidity minus bicarbonate (
TA − HCO
3
−
) as indicated
above for some of the experimental series. Measurements
of
22
Na gamma radioactivity were conducted both on
individual zebrafish carcasses and on 1- mL aliquots of
initial and final experimental water samples on a Perkin
Elmer Wallac Wizard 1480 Automatic Gamma Counter
(Waltham, MA). Water total [Na
+
]
o
and [K
+
]
o
were
measured by atomic absorption flame spectrophotom-
etry (Varian Model 1275, Mulgrave). Water T
[Amm]
and
TA − HCO
3
−
were measured as previously described to
calculate
J
H
net
.
89
4.5
|
Calculations
Rates of Na
+
uptake (
J
Na
in
; nmol g
−1
hour
−1
) were calcu-
lated as:
where
CPM
fish
is the measured counts per minute in the
fish, m is the animal mass (g), Δt is the duration of the flux
period, SA refers to mean specific activity (nmol CPM
−1
),
which was calculated as:
where
[Na
+
]
i
,
[Na
+
]
f
CPM
i
and
CPM
f
correspond to the
[Na
+
]
o
and CPMs of initial and final collected water sam-
ples. Net flux rates of total ammonia (
J
amm
net
), were calcu-
lated as:
where [Amm]
i
and [Amm]
f
refer to the total ammonia
concentration in initial and final water samples, V refers to
the flux volume and other notations correspond as above.
Analogous equations were utilized to calculate net K
+
flux
(
J
K
net
).
4.6
|
Statistical analyses
All data are presented as mean±SE. A fiducial limit of
P<.05 was set for all statistical comparisons with all sta-
tistical and regression analyses conducted using Prism 7
for Mac (Graphpad). All data were assessed to meet the
assumptions of normality and homoscedasticity prior to
being analysd using either one- way or two- way analysis of
variance (ANOVA). Data not meeting the aforementioned
assumptions were rank- transformed and reassessed
against the assumptions of ANOVA and rank- transformed
data were thereafter utilized in ANOVA assessment and
subsequent post hoc analysis. Non- parametric analysis
was utilized when assumptions were unable to be met,
with Dunnett's test applied for multiple comparisons
against a control group. Differences amongst groups were
determined via Tukey or Sidak post hoc tests where ap-
propriate. In Series 4, correlations between
J
K
net
and
J
Na
in
measured in K
+
- free conditions, and between
J
Na
in
and
[K
+
]
o
measured during control pH exposure were evalu-
ated using Pearson's correlation coefficient and linear re-
gression analysis. In these regression analyses, the slope
of the line of best fit was tested against the null hypothesis
of slope=1 (
J
K
net
versus
J
Na
in
) or slope=0 ([K
+
]
o
versus
J
Na
in
). Correlations between
J
Na
in
and [K
+
]
o
measured
(2)
J
Na
in
=
(
(CPM
fish
⋅ SA) ⋅
1
m
⋅
1
Δt
)
(3)
SA
=
(
[Na
+
]
i
CPM
i
+
[Na
+
]
f
CPM
f
)
2
(4)
J
amm
net
=
(
[Amm]
f
− [Amm]
i
)
⋅
1
m
⋅
1
Δt

18 of 20
|
CLIFFORD et al.
during either acute (0- 2hours) or prolonged (8- 10hours)
pH 4.0 exposure in series 4 were fitted to single- phase ex-
ponential decay models and the half- inhibition constants
from each curve were tested against one another with a
comparison of fits analysis. In Series 5,
J
Na
in
and
J
K
net
data were evaluated against Michaelis- Menten and linear
regression models and the most appropriate fit was deter-
mined for each treatment; differences in J
max
and K
m
pa-
rameters for
J
Na
in
data were tested using a comparison of
fits analysis, while
J
K
net
data were tested against the null
hypothesis of slope=0.
ACKNOWLEDGEMENTS
The authors thank Noah's Pet Ark for supplying zebrafish,
Patrick Tamkee and Eric Lotto for help in the UBC-
Zoology Aquatics Facility, Drs. Colin Brauner, Samuel
Starko, Scott Parks and Alex Zimmer for data discussions,
and to the three anonymous referees for their thoughtful
and constructive reviews.
CONFLICTS OF INTEREST
The authors declare no competing interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly
available on Dryad at https://doi.org/10.6076/D1KK5Z
ref. (90).
ORCID
Alexander M. Clifford https://orcid.org/0000-0002-2836-5832
Martin Tresguerres https://orcid.org/0000-0002-7090-9266
Greg G. Goss https://orcid.org/0000-0003-0786-8868
Chris M. Wood https://orcid.org/0000-0002-9542-2219
REFERENCES
1. Krogh A.Osmotic Regulation in Aquatic Animals. Cambridge:
Cambridge University Press; 1939.
2. Maetz J. Na
+
/NH
4
+
, Na
+
/H
+
exchanges and NH
3
move-
ment across the gill of Carassius auratus. J Exp Biol.
1973;58(1):255- 275.
3. Maetz J, Romeu FG. The mechanism of sodium and chloride
uptake by the gills of a fresh- water fish, Carassius auratus:
II. Evidence for NH
4
+
/Na
+
and HCO
3
−
/Cl
−
exchanges. J Gen
Physiol. 1964;47(6):1209- 1227.
4. Wright PA, Wood CM. A new paradigm for ammonia excretion
in aquatic animals: role of Rhesus (Rh) glycoproteins. J Exp
Biol. 2009;212(Pt 15):2303- 2312.
5. Wu S- C, Horng J- L, Liu S- T, et al. Ammonium- dependent sodium
uptake in mitochondrion- rich cells of medaka (Oryzias latipes)
larvae. Am J Physiol- Cell Physiol. 2009;298(2):C237- C250.
6. Nakada T, Hoshijima K, Esaki M, Nagayoshi S, Kawakami
K, Hirose S. Localization of ammonia transporter Rhcg1 in
mitochondrion- rich cells of yolk sac, gill, and kidney of zebraf-
ish and its ionic strength- dependent expression. Am J Physiol-
Regul Integr Comp Physiol. 2007;293(4):R1743- R1753.
7. Nawata CM, Hung CCY, Tsui TKN, Wilson JM, Wright PA,
Wood CM. Ammonia excretion in rainbow trout (Oncorhynchus
mykiss): evidence for Rh glycoprotein and H
+
- ATPase involve-
ment. Physiol Genomics. 2007;31(3):463- 474.
8. Dymowska AK, Schultz AG, Blair SD, Chamot D, Goss GG.
Acid- sensing ion channels are involved in epithelial Na
+
up-
take in the rainbow trout Oncorhynchus mykiss. Am J Physiol-
Cell Physiol. 2014;307(3):C255- C265.
9. Dymowska AK, Boyle D, Schultz AG, Goss GG. The role of acid-
sensing ion channels in epithelial Na
+
uptake in adult zebrafish
(Danio rerio). J Exp Biol. 2015;218(8):1244- 1251.
10. Avella M, Bornancin M. A new analysis of ammonia and so-
dium transport through the gills of the freshwater rainbow
trout (Salmo gairdneri). J Exp Biol. 1989;142:155- 217.
11. Wilson JM, Randall DJ, Donowitz M, Vogl AW, Ip AK.
Immunolocalization of ion- transport proteins to bran-
chial epithelium mitochondria- rich cells in the mudskip-
per (Periophthalmodon schlosseri). J Exp Biol. 2000;203(Pt
15):2297- 2310.
12. Sullivan G, Fryer J, Perry S. Immunolocalization of proton
pumps (H
+
- ATPase) in pavement cells of rainbow trout gill. J
Exp Biol. 1995;198(12):2619- 2629.
13. Kwong RWM, Perry SF. A role for sodium- chloride cotransport-
ers in the rapid regulation of ion uptake following acute envi-
ronmental acidosis: new insights from the zebrafish model. Am
J Physiol- Cell Physiol. 2016;311(6):C931- C941.
14. Wu Shu- Chen, Horng Jiun- Lin, Liu Sian- Tai, et al. Ammonium-
dependent sodium uptake in mitochondrion- rich cells of
medaka (Oryzias latipes) larvae. Am J Physiol- Cell Physiol.
2010;298(2):C237– C250.
15. Yang T, Huang YG, Singh I, Schnermann J, Briggs JP.
Localization of bumetanide- and thiazide- sensitive Na- K- Cl
cotransporters along the rat nephron. Am J Physiol- Ren Physiol.
1996;271(4):F931- F939.
16. Jacquillet G, Rubera I, Unwin RJ. Potential role of serine pro-
teases in modulating renal sodium transport in vivo. Nephron
Physiol. 2011;119(2):p22- p29.
17. Hirata T, Kaneko T, Ono T, et al. Mechanism of acid adaptation
of a fish living in a pH 3.5 lake. Am J Physiol- Regul Integr Comp
Physiol. 2003;284(5):R1199- R1212.
18. Esaki M, Hoshijima K, Kobayashi S, Fukuda H, Kawakami K,
Hirose S. Visualization in zebrafish larvae of Na
+
uptake in
mitochondria- rich cells whose differentiation is dependent on
foxi3a. Am J Physiol- Regul Integr Comp Physiol. 2007;292(1):R4
70- R480.
19. Yan J- J, Chou M- Y, Kaneko T, Hwang P- P. Gene expression of
Na
+
/H
+
exchanger in zebrafish H
+
- ATPase- rich cells during
acclimation to low- Na
+
and acidic environments. Am J Physiol-
Cell Physiol. 2007;293(6):C1814- C1823.
20. Parks SK, Tresguerres M, Goss GG. Theoretical consid-
erations underlying Na
+
uptake mechanisms in freshwa-
ter fishes. Comp Biochem Physiol C- Toxicol Pharmacol.
2008;148(4):411- 418.
21. Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in
the wild: a review of natural history and new notes from the
field. Zebrafish. 2007;4(1):21- 40.
22. Shamshuddin J, Panhwar QA, Alia FJ, Fauziah CI.
Formation and utilisation of acid sulfate soils in Southeast
Asia for sustainable rice cultivation. Trop Agric Sci.
2017;40(2):225- 246.

|
19 of 20
CLIFFORD et al.
23. Morales LA, Paz- Ferreiro J, Vieira SR, Vázquez EV. Spatial and
temporal variability of Eh and pH over a rice field as related to
lime addition. Bragantia. 2010;69:67- 76.
24. Azura A, Shamshuddin J, Fauziah CI. Root elongation, root
surface area and organic acid by rice seedling under Al
3+
and/
or H
+
stress. Am J Agric Biol Sci. 2011;6(3):321- 331.
25. Shamshuddin J, Elisa AA, Ali R, Fauziah I. Rice defense mech-
anisms against the presence of excess amount of Al
3+
and Fe
2+
in the water. Aust J Crop Sci. 2013;7(3):314- 320.
26. Alia FJ, Shamshuddin J, Fauziah CI, Husni MHA, Panhwar
QA. Effects of Aluminum, Iron and/or low pH on rice
seedlings grown in solution culture. Int J Agric Biol.
2015;17(4):702- 710.
27. Sundin J, Morgan R, Finnøen MH, Dey A, Sarkar K, Jutfelt F.
On the observation of wild zebrafish (Danio rerio) in India.
Zebrafish. 2019;16(6):546- 553.
28. Lewis L, Kwong RWM. Zebrafish as a model system for inves-
tigating the compensatory regulation of ionic balance during
metabolic acidosis. Int J Mol Sci. 2018;19(4):1087.
29. Kumai Y, Perry SF. Ammonia excretion via Rhcg1 facilitates
Na
+
uptake in larval zebrafish, Danio rerio, in acidic water. Am
J Physiol- Regul Integr Comp Physiol. 2011;301(5):R1517- R1528.
30. Kumai Y, Bernier NJ, Perry SF. Angiotensin- II promotes Na
+
uptake in larval zebrafish, Danio rerio, in acidic and ion- poor
water. J Endocrinol. 2014;220(3):195- 205.
31. Caner T, Abdulnour- Nakhoul S, Brown K, Islam MT, Hamm
LL, Nakhoul NL. Mechanisms of ammonia and ammonium
transport by rhesus- associated glycoproteins. Am J Physiol- Cell
Physiol. 2015;309(11):C747- C758.
32. Zimmer AM, Shir- Mohammadi K, Kwong RWM, Perry SF.
Reassessing the contribution of the Na
+
/H
+
exchanger Nhe3b
to Na
+
uptake in zebrafish (Danio rerio) using CRISPR/Cas9
gene editing. J Exp Biol. 2020;223:jeb215111.
33. Chang W- J, Wang Y- F, Hu H- J, Wang J- H, Lee T- H, Hwang P-
P. Compensatory regulation of Na
+
absorption by Na
+
/H
+
ex-
changer and Na
+
- C l
-
cotransporter in zebrafish (Danio rerio).
Front Zool. 2013;10(1):46.
34. Kleyman TR, Cragoe EJ. Amiloride and its analogs as tools in
the study of ion transport. J Membr Biol. 1988;105(1):1- 21.
35. Boyle D, Clifford AM, Orr E, Chamot D, Goss GG. Mechanisms
of Cl
-
uptake in rainbow trout: Cloning and expression of
slc26a6, a prospective Cl
-
/HCO
3
-
exchanger. Comp Biochem
Physiol - Mol Integr Physiol. 2015;180:43- 50.
36. Chen X, Qiu L, Li M, et al. Diarylamidines: high potency in-
hibitors of acid- sensing ion channels. Neuropharmacology.
2010;58(7):1045- 1053.
37. Brix KV, Brauner CJ, Schluter D, Wood CM. Pharmacological
evidence that DAPI inhibits NHE2 in Fundulus heteroclitus
acclimated to freshwater. Comp Biochem Physiol Part C Toxicol
Pharmacol. 2018;211:1- 6.
38. Shih T- H, Horng J- L, Liu S- T, Hwang P- P, Lin L- Y. Rhcg1 and
NHE3b are involved in ammonium- dependent sodium up-
take by zebrafish larvae acclimated to low- sodium water. Am J
Physiol Regul Integr Comp Physiol. 2012;302(1):R84- 93.
39. Garvin JL, Simon SA, Cragoe EJ, Mandel LJ. Phenamil: An irre-
versible inhibitor of sodium channels in the toad urinary blad-
der. J Membr Biol. 1985;87(1):45- 54.
40. Alvarez de la Rosa D, Canessa CM, Fyfe GK, Zhang P. Structure
and regulation of amiloride- sensitive sodium channels. Annu
Rev Physiol. 2000;62:573- 594.
41. Reid SD, Hawkings GS, Galvez F, Goss GG. Localization
and characterization of phenamil- sensitive Na
+
influx
in isolated rainbow trout gill epithelial cells. J Exp Biol.
2003;206(Pt 3):551- 559.
42. Giménez I. Molecular mechanisms and regulation of
furosemide- sensitive Na– K– Cl cotransporters. Curr Opin
Nephrol Hypertens. 2006;15(5):517.
43. Stokes J, Lee I, Damico M. Sodium- chloride absorption
by the urinary- bladder of the winter flounder - a thiazide-
sensitive, electrically neutral transport- system. J Clin Invest.
1984;74(1):7- 16.
44. Beaumont K, Vaughn DA, Maciejewski AR, Fanestil DD.
Reversible downregulation of thiazide diuretic receptors by
acute renal ischemia. Am J Physiol- Ren Physiol. 1989;256(2):F3
29- F334.
45. Wang Y- F, Tseng Y- C, Yan J- J, Hiroi J, Hwang P- P. Role of
SLC12A10.2, a Na- Cl cotransporter- like protein, in a Cl up-
take mechanism in zebrafish (Danio rerio). Am J Physiol- Regul
Integr Comp Physiol. 2009;296(5):R1650- R1660.
46. Horng J- L, Hwang P- P, Shih T- H, Wen Z- H, Lin C- S, Lin L- Y.
Chloride transport in mitochondrion- rich cells of euryhaline
tilapia (Oreochromis mossambicus) larvae. Am J Physiol- Cell
Physiol. 2009;297(4):C845- C854.
47. Maren TH. Carbonic anhydrase: chemistry, physiology, and in-
hibition. Physiol Rev. 1967;47(4):595- 781.
48. Yellen G. Permeation in potassium channels: Implications for
channel structure. Annu Rev Biophys Chem. 1987;16(1):227- 246.
49. Taglialatela M, Drewe JA, Brown AM. Barium blockade of a
clonal potassium channel and its regulation by a critical pore
residue. Mol Pharmacol. 1993;44(1):180- 190.
50. Choe H, Sackin H, Palmer LG. Permeation properties of inward-
rectifier potassium channels and their molecular determinants.
J Gen Physiol. 2000;115(4):391- 404.
51. Rubino JG, Wilson JM, Wood CM. An in vitro analysis of in-
testinal ammonia transport in fasted and fed freshwater rain-
bow trout: roles of NKCC, K
+
channels, and Na
+
, K
+
ATPase. J
Comp Physiol B. 2019;189:549- 566.
52. Wu Z- Z, Li D- P, Chen S- R, Pan H- L. Aminopyridines poten-
tiate synaptic and neuromuscular transmission by targeting
the voltage- activated calcium channel β subunit. J Biol Chem.
2009;284(52):36453- 36461.
53. Latorre R, Vergara C, Hidalgo C. Reconstitution in planar lipid
bilayers of a Ca
2+
- dependent K
+
channel from transverse tu-
bule membranes isolated from rabbit skeletal muscle. Proc Natl
Acad Sci USA. 1982;79(3):805- 809.
54. Lang DG, Ritchie AK. Tetraethylammonium blockade of
apamin- sensitive and insensitive Ca
2+
- activated K
+
channels in
a pituitary cell line. J Physiol. 1990;425:117- 132.
55. Khodakhah K, Melishchuk A, Armstrong CM. Killing K channels
with TEA+. Proc Natl Acad Sci USA. 1997;94(24):13335- 13338.
56. Zemková H, Teisinger J, Vyskocil F. Inhibition of the electro-
genic Na, K pump and Na, K- ATPase activity by tetraethylam-
monium, tetrabutylammonium, and apamin. J Neurosci Res.
1988;19(4):497- 503.
57. Kim M- H, Korogod N, Schneggenburger R, Ho W- K, Lee
S- H. Interplay between Na
+
/Ca
2+
exchangers and mito-
chondria in Ca
2+
clearance at the Calyx of Held. J Neurosci.
2005;25(26):6057- 6065.
58. Lee JS, Kim M- H, Ho W- K, Lee S- H. Developmental up-
regulation of presynaptic NCKX underlies the decrease of

20 of 20
|
CLIFFORD et al.
mitochondria- dependent posttetanic potentiation at the rat
calyx of Held synapse. J Neurophysiol. 2013;109(7):1724- 1734.
59. Kumai Y, Bahubeshi A, Steele S, Perry SF. Strategies for main-
taining Na
+
balance in zebrafish (Danio rerio) during prolonged
exposure to acidic water. Comp Biochem Physiol A Mol Integr
Physiol. 2011;160(1):52- 62.
60. Brix KV, Grosell M. Comparative characterization of Na
+
transport in Cyprinodon variegatus and Cyprinodon variegatus
hubbsi: a model species complex for studying teleost invasion of
freshwater. J Exp Biol. 2012;215(7):1199- 1209.
61. Tresguerres M, Clifford AM, Harter TS, et al. Evolutionary links
between intra- and extracellular acid– base regulation in fish
and other aquatic animals. J Exp Zool Part Ecol Integr Physiol.
2020;333(6):449- 465.
62. Hwang PP, Lee TH. New insights into fish ion regulation
and mitochondrion- rich cells. Comp Biochem Physiol Part A.
2007;148:479- 497.
63. Kirschner LB. Water and ions. In: Prosser CL, ed. Environmental
and metabolic animal physiology. 1991:13- 107.
64. Wood CM, LeMoigne J. Intracellular acid- base responses to
environmental hyperoxia and normoxic recovery in rainbow
trout. Respir Physiol. 1991;86(1):91- 113.
65. Morgan IJ, Potts WTW, Oates K. Intracellular ion concen-
trations in branchial epithelial cells of brown trout (Salmo
trutta L.) determined by X- ray microanalysis. J Exp Biol.
1994;194(1):139- 151.
66. García Romeu F, Maetz J. The mechanism of sodium and chlo-
ride uptake by the gills of a fresh- water fish, Carassius auratus.
J Gen Physiol. 1964;47(6):1195- 1207.
67. Gonzalez RJ, Wood CM, Wilson RW, et al. Effects of water pH
and calcium concentration on ion balance in fish of the Rio
Negro. Amazon. Physiol Zool. 1998;71(1):15- 22.
68. Wilson RW, Wood CM, Gonzalez RJ, et al. Ion and acid- base balance
in three species of Amazonian fish during gradual acidification of
extremely soft water. Physiol Biochem Zool. 1999;72(3):277- 285.
69. Evans DH. Osmotic and Ionic Regulation: Cells and Animals, 1st
ed. CRC Press; 2008.
70. Wood CM, Bucking C. 5 - The role of feeding in salt and
water balance. In: Grosell M, Farrell AP, Brauner CJ, eds. Fish
Physiology. Academic Press; 2010:165- 212.
71. Furukawa F, Watanabe S, Kimura S, Kaneko T. Potassium ex-
cretion through ROMK potassium channel expressed in gill
mitochondrion- rich cells of Mozambique tilapia. Am J Physiol-
Regul Integr Comp Physiol. 2011;302(5):R568- R576.
72. Neyton J, Miller C. Potassium blocks barium permeation
through a calcium- activated potassium channel. J Gen Physiol.
1988;92(5):549- 567.
73. Ma X- Y, Yu J- M, Zhang S- Z, et al. External Ba
2+
block of the
two- pore domain potassium channel TREK- 1 defines con-
formational transition in its selectivity filter. J Biol Chem.
2011;286(46):39813- 39822.
74. Judge SIV, Bever CT. Potassium channel blockers in multiple
sclerosis: neuronal K
v
channels and effects of symptomatic
treatment. Pharmacol Ther. 2006;111(1):224- 259.
75. He C, O’Halloran DM. Analysis of the Na
+
/Ca
2+
exchanger
gene family within the phylum Nematoda. PLoS One.
2014;9(11):e112841.
76. Su YH, Vacquier VD. A flagellar K
+
- dependent Na
+
/Ca
2+
ex-
changer keeps Ca
2+
low in sea urchin spermatozoa. Proc Natl
Acad Sci USA. 2002;99(10):6743- 6748.
77. Sakurai K, Vinberg F, Wang T, Chen J, Kefalov VJ. The Na
+
/
Ca
2+
, K
+
exchanger 2 modulates mammalian cone phototrans-
duction. Sci Rep. 2016;6(1):32521.
78. Lamason RL, Mohideen M- APK, Mest JR, et al. SLC24A5, a pu-
tative cation exchanger, affects pigmentation in zebrafish and
humans. Science. 2005;310(5755):1782- 1786.
79. Hassan MT, Lytton J. Potassium- dependent sodium- calcium
exchanger (NCKX) isoforms and neuronal function. Cell
Calcium. 2020;86:102135.
80. Lee S- H, Kim M- H, Park KH, Earm YE, Ho W- K. K
+
- dependent
Na
+
/Ca
2+
exchange is a major Ca
2+
clearance mechanism in
axon terminals of rat neurohypophysis. J Neurosci Off J Soc
Neurosci. 2002;22(16):6891- 6899.
81. Altimimi HF, Schnetkamp PPM. Na
+
/Ca
2+
- K
+
exchang-
ers (NCKX) - Functional properties and physiological roles.
Channels. 2007;1(2):62- 69.
82. Altimimi HF, Szerencsei RT, Schnetkamp PPM. Functional and
structural properties of the NCKX2 Na
+
/Ca
2+
- K
+
exchanger: a
comparison with the NCX1 Na
+
/Ca
2+
exchanger. Adv Exp Med
Biol. 2013;961:81- 94.
83. Yang H, Choi K- C, Jung E- M, An B- S, Hyun S- H, Jeung E- B.
Expression and regulation of sodium/calcium exchangers,
NCX and NCKX, in reproductive tissues: do they play a critical
role in calcium transport for reproduction and development?
Adv Exp Med Biol. 2013;961:109– 121.
84. Schnetkamp PPM. The SLC24 gene family of Na
+
/Ca
2+
– K
+
ex-
changers: From sight and smell to memory consolidation and
skin pigmentation. Mol Aspects Med. 2013;34(2- 3):455- 464.
85. Kwong RWM, Kumai Y, Perry SF. The physiology of fish
at low pH: the zebrafish as a model system. J Exp Biol.
2014;217(Pt 5):651- 662.
86. Gonzalez RJ, Patrick ML, Duarte RM, et al. Exposure to pH 3.5 water
has no effect on the gills of the Amazonian tambaqui (Colossoma
macropomum). J Comp Physiol [B]. 2021;191(3):493- 502.
87. Morris C, Val AL, Brauner CJ, Wood CM. The physiology of fish
in acidic waters rich in dissolved organic carbon, with specific
reference to the Amazon basin: Ionoregulation, acid– base reg-
ulation, ammonia excretion, and metal toxicity. J Exp Zool Part
Ecol Integr Physiol. 2021;335(9- 10):843– 863.
88. Dymowska AK, Hwang P- P, Goss GG. Structure and function
of ionocytes in the freshwater fish gill. Respir Physiol Neurobiol.
2012;184(3):282- 292.
89. Clifford AM, Weinrauch AM, Goss GG. Dropping the
base: Recovery from extreme hypercarbia in the CO
2
tol-
erant Pacific hagfish (Eptatretus stoutii). J Comp Physiol B.
2018;188(3):421- 435.
90. Clifford A, Tresguerres M, Goss G, Wood C. Dataset for ‘A novel
K
+
- dependent Na
+
uptake mechanism during low pH expo-
sure in adult zebrafish (Danio rerio): New tricks for old dogma’,
Dryad, Dataset, https://doi.org/10.6076.D1KK5Z.
How to cite this article: Clifford AM, Tresguerres
M, Goss GG, Wood CM. A novel K
+
- dependent Na
+
uptake mechanism during low pH exposure in
adult zebrafish (Danio rerio): New tricks for old
dogma. Acta Physiol. 2022;00:e13777. doi:10.1111/
apha.13777
