
1
5
6
4
3
2
Department of Health
Best practice guide
to clinical incident
management
Second edition – January 2023

Best practice guide to clinical incident management
Second edition – January 2023
Published by the State of Queensland (Queensland Health) January 2023
This Guide is licensed under a Creative Commons Attribution 3.0 Australian licence.
To view a copy of this licence, visit www.creativecommons.org.au/learn/licences
© State of Queensland (Department of Health) 2023
You are free to copy, communicate and adapt the work, providing you attribute the State of Queensland
(Department of Health)
For more information contact:
Patient Safety and Quality
Clinical Excellence Queensland
PO Box 2368
Fortitude Valley BC 4006
Australia
An electronic version of this Guide is available at http://qheps.health.qld.gov.au/psu
Disclaimer:
The content presented in this publication is distributed by the Queensland Government as an
information source only. The State of Queensland makes no statement, representation or warranties
about the accuracy, completeness of reliability of any information contained in this publication. The
State of Queensland disclaims all responsibility and liability (including without limitation for liability in
negligence) for all expenses, losses, damages and costs you may incur because of the information being
inaccurate or incomplete in any way, and for any reason reliance was placed on such information.

Contents | 3
Contents
List of gures 6
List of tables 6
Foreword 7
Introduction ................................................................................................................................... 8
Introduction and background 9
Purpose of this Guide 10
Scope of the Guide 11
Target audience 11
Timeline of accident/incident analysis methods and models 12
Patient, family, carer partnership ................................................................................................... 13
Involving the patient, family and/or carer 14
Person-centred care 14
Immediate response or unexpected situations 14
Clinician disclosure 15
Open disclosure 15
The analysis—what, how and why it happened 15
Following the analysis 17
Partners in building trusting relationships 17
Clinical Incident Managment Process ............................................................................................. 18
Clinical incident management 19
The investigation and review methodology 19
System based analysis review versus accountability review 20
Investigation legislation 20
Step 1: Before the incident 22
Key principles 22
Ensure leadership support 23
The importance of a strong patient safety culture 24
A just culture approach 25
Applying a Restorative Just Culture (RJC) 25
What are the goals of a restorative just culture? 26
Key concepts 26
Step 2: Immediate response 31
Care for and support of patient, family, carer, clinicians and others 31
Report incident 31
Secure items 32
Begin the disclosure process 32
Reduce risk of imminent recurrence 33
Step 3: Prepare for analysis 34
Preliminary assessment 34
Methods of incident analysis - overview 35
Selecting a method of incident analysis 35
Severity/probability matrix score 37
Level/type of analysis based on the degree of harm 37
Identify the team and the team approach 38
Coordinate meetings 39
Plan for and conduct interviews 39
Avoiding cognitive traps 40

4 | Best practice guide to clinical incident management Second edition - January 2023
Contents continued
Step 4: Analysis process 42
Comprehensive analysis 43
Steps in conducting a comprehensive analysis 44
What happened? 44
How and why it happened 45
Use systems theory and human factors 46
Using diagramming 47
Summarise ndings 49
What can be done to reduce the risk of recurrence and make care safer? 49
What was learned 49
Concise analysis 50
When to use a Concise approach 52
Steps in conducting a concise analysis 53
What happened 53
How and why it happened 53
What can be done to reduce the risk of recurrence and make care safer? 53
What was learned 54
Multi-incident analysis 55
Examples of multi-incident analysis 57
Steps in conducting a multi-incident analysis 58
What happened 58
How and why it happened 59
What can be done to reduce the risk of recurrence and make care safer? 59
What was learned 59
Recommended actions 60
Develop and manage recommended actions 60
Key features of eective recommended actions 60
Suggest an order of priority for recommended actions 63
Strength of recommendation/s 64
Consult on the dra recommended actions 65
Prepare and hand-over report 66
Manage recommended actions 66
Validate actions from strategic and operational perspectives 66
Delegate recommended actions for implementation and empower implementation 67
Step 5: Follow through 68
Implement recommended actions 68
Monitor and assess the eectiveness of recommended actions 70
Step 6: Close the loop 72
Close the loop 72
Continuous organisational learning and sharing results 72
Reflecting on and improving the quality of analysis and management processes 73
Conclusion 74

Contents | 5
Contents continued
Appendices....................................................................................................................................75
Appendix A – Analysis team membership, roles and responsibilities 77
Appendix B – Incident reporting and investigation legislation 79
Appendix C – A just culture approach 82
Appendix D – Restorative just culture framework
Appendix E – Creating a constellation diagram 84
Appendix F – Severity assessment code (SAC) matrix 89
Appendix G – Guide to level/type of analysis 90
Appendix H – Sample analysis team charter 91
Appendix I – Team management checklist 92
Appendix J – Investigative interview guidance (cognitive type interview) 93
Appendix K – Case study—comprehensive analysis: 95
resident absconds from a residential aged care facility
Appendix L – Incident analysis guiding questions 103
Appendix M – Three human factors methods that can be used in incident analysis 106
Appendix N – Developing a statement of ndings template and examples 109
Appendix O – Case study—concise analysis: medication incident 111
Appendix P – Lessons learned 117
Glossary ................................................................................................................................... 119
References ...................................................................................................................................122

6 | Best practice guide to clinical incident management Second edition - January 2023
List of gures
Figure 1. Timeline of methods and models 1900–2030 12
Figure 2. Clinical Incident Management Process Steps 19
Figure 3. Complex, complicated, simple systems matrix 29
Figure 4. System levels 30
Figure 5. Flow diagram for comprehensive analysis 43
Figure 6. Ishikawa diagram 47
Figure 7: Tree diagram 47
Figure 8. Example of a constellation diagram 48
Figure 9. Flow diagram for concise analysis 51
Figure 10. Flow diagram for multi analysis 56
Figure 11. Hierarchy of eectiveness 61
Figure 12. Impact and Achievability Matrix 65
Figure 13. Run chart 71
List of tables
Table 1. Clinical Incident Management Principles
22
Table 2. Safety I and Safety II concepts 26
Table 3. Criteria to consider in selecting an incident analysis method 36
Table 4. Severity versus probability matrix
Table 5. Characteristics of concise and comprehensive incident analysis 52
Table 6. SMARTER format 61
Table 7. Risk assessment matrix 63
Table 8. Example of table to summarise and prioritise recommended actions 64
Table 9. Strength of recommendations eect and eort actions 65
Table 10. Example of a tool to track the implementation status of recommended actions 67
Table 11. Key questions in designing data collection 71
Table 12. Patient Safety Notications (Alerts, Notices, Communiques) 7

Foreword
The Best Practice Guide to Clinical Incident
Management has been developed as a
statewide resource to support Queensland
Health sta responsible for, or involved in
managing, analysing and learning from patient
safety incidents in healthcare settings. The
aim is to foster safe and reliable care, reduce
preventable incidents and improve patient
safety outcomes. This revised edition of
the Best Practice Guide to Clinical Incident
Management (the Guide) responds to changes
in methodology and approaches that have
occurred in clinical incident management,
nationally and internationally since it was rst
published in 2014.
Queensland Health is committed to improving
patient safety, through the review of
contemporary literature review, instigating
changes to relevant legislation, promoting
adherence to National Safety and Quality Health
Service Standards second edition and providing
strong health service leadership with a focus on
creating positive safety cultures.
The health environment in which we provide
care, by its very nature, poses potential risk
across the spectrum of patient services. We must,
therefore, learn from potential and actual patient
harm scenarios, without fear of blame, if we are
to reduce future harm. This new edition strongly
emphasises the need to embed a Restorative
Just Culture when responding to incidents.
The framework for a Restorative Just Culture
is embedded in this Guide and is central to
enabling a patient-centric approach: it replaces a
backward-looking determination with a forward-
looking review of the clinical incident engaging
participation by all stakeholders, including the
sta who may be second victims, to address the
harms and causes for improvements.
To further strengthen the clinical analysis
process in Queensland Health, the Patient
Safety Health Service Directive Guideline for
Clinical Incident Management includes a new
section for the development and implementation
of recommendations. Ensuring the right
stakeholders are involved in the development of
recommendations is essential. It is also critical to
ensure that the developed recommendations are
eectively implemented and sustainable: a step
by step process has been outlined in this edition
to assist health services. Lesson learned that are
well documented and widely shared will improve
work processes, enhance quality and safety, and
build resilient systems to prevent recurrences.
With improvements and changes to Queensland
Health legislation, the Patient Safety and Quality,
Clinical Excellence Queensland (PSQ,CEQ) is now
able to share Severity Assessment Code 1 (SAC1)
clinical analysis reports with Quality Assurance
Committees to establish a shared understanding
of local and statewide gaps in clinical incident
management and governance. This will provide
enhanced opportunities for sustainable system
wide improvements.
I would like to acknowledge the work of the
World Health Organisation (WHO) Patient Safety
Program, the Canadian Patient Safety Institute
and National Health Service (NHS) in the
foundational work of this Guide. Since this Guide
was initially developed in 2014, there have been
signicant advances in the way clinical incidents
are identied and reviewed to inform patient
safety practice and quality improvement, both
nationally and internationally. The Australian
Commission on Safety and Quality in Health Care
(the Commission) is recognised for their role in
advancing health care standards, promoting
patient safety and the development of a broad
range of contemporary resources to improve the
quality of health care provision.
I am pleased to be able to present this Guide as
a key statewide resource to further enhance the
eectiveness of clinical incident management
by incorporating the practical aspects of
involving the patient and family or carer,
conducting an analysis, developing a report and
recommendations, implementing and sustaining
continuous improvements, and sharing the
lessons learnt in a safe and just culture. This
Guide should be read in conjunction with the
Open Disclosure Guide, 2020, along with the
range of Queensland Health clinical incident
management resources and used in conjunction
with other resources that support organisations
to achieve National Safety and Quality Health
Service Standards Implementation.
Kirstine Sketcher-Baker
Executive Director
Patient Safety and Quality
Clinical Excellence Queensland
Foreword | 7

Introduction

Introduction | 9
Introduction and background
Everyday across Queensland Health’s hospital
and health services, patients receive high
quality, safe and eective care from skilled
clinicians that is delivered in demanding, highly
complex and busy environments. However,
despite our best eorts, clinical incidents do
occasionally occur. These range from near
misses to those that cause temporary harm to
permanent harm or death to patients. When
these incidents occur, it is distressing for
patients, their families or carers and for the sta
involved. To prevent these incidents occurring
again, it imperative that the incident is reported
into the RiskMan incident management system
as part of a patient safety culture of incident
reporting. This will enable sta to respond
eectively through an investigation process
including the identication and implementation
of improvements.
As per the Health Service Directive Patient
Safety, issued under Section 47 of the
Hospital and Health Boards Act 2011 (HHB
Act), Queensland Health is required to have a
clinical incident management process in place
to manage all clinical incidents, and sta are
mandated to report SAC1 clinical incidents.
Healthcare sta are mandated to report to
the PSQ, CEQ through Queensland Health’s
RiskMan information system within one day
of becoming aware of the SAC1 event: utilising
Queensland Health’s incident management
systems are crucial to providing safe care.
It is imperative that the patients and families or
carers, and the sta who care for them are fully
supported, informed, and involved when any
type of health care related incident occurs. The
impact associated with a clinical incident may
extend for months and even years, aecting
personal health, relationships and careers.
Feelings of anger, frustration and complicated
grieving may result
(1)
when communication
and information is not eectively managed
and where there are gaps in learning and
improvement.
To purposefully manage this, disclosure
processes form a key step in clinical incident
management and importantly commence upon
the identication of a patient safety incident.
Queensland Health actively promotes the
organisation-wide use of Clinician Disclosure
where the treating clinician informs the patient
of what has occurred, apologises and advises
the patient of the next steps. In response to a
SAC1 and/or SAC2 clinical incident, a higher-
level response (Formal Open Disclosure) may
be required, in addition to the initial step of
undertaking Clinician Disclosure.
Previous theory relating to investigating
incidents focused on the role of human error.
Contemporary research has determined
that most incidents and accidents are due
to a failure within the system.
(2)
Adopting a
systems approach to understanding incidents
is the preferred framework for the analysis
of clinical incidents, either retrospectively or
prospectively, whilst paying attention to human
factors sciences. Further to this, it is now widely
understood that a culture of safety, in which
sta are encouraged to report incidents and
learn from them, is essential to transforming our
health care environment. This can be achieved
through the use of transparent reporting,
objective clinical analysis insights and the
formulation of recommendations that achieve
sustainable and measurable improvements.
(3)
This objective can be achieved through both
proactive and reactive processes that:
• identify and treat risks/hazards before they
lead to patient harm (pro-active)
• identify when patients are harmed and
promptly intervene to minimise the harm
caused to a patient as a result of the incident
(reactive)
• disclose a clinical incident resulting in
patient harm (pro-active and reactive)
• ensure that lessons learned from clinical
incidents are communicated and applied
by taking preventive actions designed
to minimise the risk of similar incidents
occurring in the future (pro-active and
reactive).

10 | Best practice guide to clinical incident management Second edition - January 2023
The aim of clinical incident management is to
eectively incorporate improved and updated
approaches to managing clinical incidents, with
the view to proactively reducing preventable
patient harm. The value of implementing
clinical incident management processes in a
safety-aware culture, is now, more than ever
an essential component of achieving quality
patient care outcomes.
The importance of culture in safety and
quality improvement is articulated in a range
of the Commission’s work, including the
National Safety and Quality Health Service
(NSQHS) Standards, the National Model
Clinical Governance Framework and the
Communicating for Safety Program. Key aspects
of a positive patient safety culture include
a shared importance of safety, constructive
communication, mutual trust, an engaged
workforce, acknowledgement at all levels that
things can go wrong and the ability to recognise,
respond to, and give feedback about, and learn
from, adverse events.
(4)
Implementing systems to ensure that patient
safety incidents are recognised, reported and
analysed and information used to improve
safety systems, is a mandatory requirement of
the NSQHS Standards and are articulated under
the Australian Health Service Safety and Quality
Accreditation Scheme.
(5)
The Standards describe
a level of care that consumers can expect from
health service organisations.
The Clinical Governance Standard incorporates
criteria relating to:
• Governance, leadership and culture
• Patient safety and quality systems
• Clinical performance and eectiveness
• Safe environment for the delivery of care.
(6)
Clinical Governance Standard
Purpose of this Guide
This Guide is a resource to help support
individual and organisational learning and
to drive quality improvement, in response to
patient safety incidents. Quality improvement is
an ongoing process. This means that activities
aimed at minimising risk to patients, carers,
healthcare sta and the organisation will
be continually in various stages of review,
improvement planning and implementation. Key
aspects within the quality improvement review
process include understanding:
• what happened
• how and why it happened
• what can be done to reduce the risk of
recurrence and to make healthcare safer
• what was learned
• how the learning can be shared.
Organisations may choose to use the Guide to
support quality assurance processes. A quality
assurance mechanism assists to test whether
relevant systems are in place and ensure that
expected standards of quality and safety are in
place including:
• how incidents are recognised and reported
• how patients and/or their carers express
their concerns or incidents
• how sta and patients and/or carers are
involved in incident review
• how feedback is provided from incident
analysis review to improve safety and quality
• how risks are managed
• how incident management systems can be
more eective.
Organisations may also choose to use this
Guide to support a safety and quality culture by:
• enhancing the safety and quality of patient
care
• promoting a culture of safety and learning
within the organisation
• promoting patient and family-centred care
• encouraging learning and dissemination of
learning within and beyond the organisation
increasing the eectiveness of incident
management
• improving the success of incident analysis
as a tool in preventing and/or mitigating
harm.

Introduction | 11
Scope of the Guide
This Guide is based on key Queensland Health
Departmental Directives and Guidelines,
and provides a framework of best practice
approaches and practical tools that may be
adopted or adapted to meet local hospital
and health service circumstances and needs.
This Guide should be read in conjunction with
relevant legislation and guidelines, including
but not limited to, the following Queensland
Health and the Commission’s governance
documents:
• Hospital and Health Boards Act 2011
• Hospital and Health Regulation 2012
• Health Service Directive, Patient Safety
QH-HSD-033:2014
• Health Service Directive, Guideline for
Clinical Incident Management
QH-HSDGDL-032-2
• Queensland Health Open Disclosure Guide
2020
• Australian Commission on Safety and Quality
in Health Care- National Safety and Quality
Health Service Standards second edition
• Australian Commission on Safety and Quality
in Health Care - Australian Open Disclosure
Framework.
Target audience
This Guide is designed to be used by those
responsible for, or involved in analysing,
managing and/or learning from patient safety
incidents in any healthcare setting.
The sections including the introduction, the
patient, family and carer and Step 1 of the
clinical incident management process are
recommended reading for executives, senior
managers and safety and quality ocers.
The principles that underpin best practice for
eective incident management, are described in
the Step 1 of the clinical incident management
process.
The clinical incident management process
comprises six steps and these sections are
recommended reading for safety and quality
ocers and their line managers focusing on
practical suggestions and tools for incident
analysis and learning.

12 | Best practice guide to clinical incident management Second edition - January 2023
Timeline of accident/incident analysis methods and models
The methods now used to analyse and manage incidents have evolved, from their early beginnings in
industries such as aviation
(7)
, to reflect the unique characteristics associated with complex healthcare
systems. The value of applying dierent methods or models to investigate/analyse an incident is now
well recognised. Figure 1 below shows the development of some methods from the early 1900s.
(8,9,10)
Figure 1. Timeline of methods and models 1900–2030
1900 1910 1920 1930 1940 1950 1960 1970 1980 1990 2000 2010 2020 2030
5
Behaviourism Systemism
Cognitivism
Time &
Motion
(1911)
Action
research
(Lewin, 1944)
STEP
(Hendrick &
Brenner 1987)
RMF
(Rasmussen,
1997)
HEAPS
(ErroMed,
2001)
RCA
2
(NPSF, 2015)
Swiss
Cheese
(Reason,
1990)
HFACS
(Shappell &
Wiegmann,
2001)
EAST-BL
(Stanton &
Harvey, 2017)
STAMP
CAST
(Leveson,
2004)
FRAM
(Hollinagel,
2012)
AcciMap
(Rasmussen,
1997)
RCA in
healthcare
(1990s)
CWA
(Rasmussen,
1960s)
–
1958)
RCA - 5
Whys
(Toyoda,
1958)
–
FMEA
(late
1950s)
–
HAZOP
(ICI, late
1960s)
FTA
(Watson,
1961)
Bow-Tie
(ICI,
1979)
PRA/PSA
(late
1960s)
CSE
(Woods &
Hollnagel,
1982)
Adapted from Hollnagel (2012), Waterson et al (2015) and Stanton et al (2019)
Age of technology
Age of human factors
Age of complex sociotechnical systems
PRS/PSA Probabilistic risk assessment / Probabilistic safety assessment
FTA Fault tree analysis
RCA Root cause analysis
RCA 2 Root cause analysis and action
HAZOP Hazard and operability study
FMEA Failure modes and eects analysis
STEP Sequentially timed events plotting
CSE/CWA Cognitive systems engineering / Cognitive work analysis
RMF Risk management framework
STAMP Systems theoretic accident modelling and processes
CAST Causal analysis based on STAMP
HEAPS Human error and patient safety
HFACS Human factors and classication system
FRAM Functional Resonance Analysis Method
EAST-BL Event Analysis of Systemic Teamwork Broken Links

Patient, family,
carer partnership

14 | Best practice guide to clinical incident management Second edition - January 2023
Involving the patient, family
and/or carer
The NSQHS Partnering with Consumers Standard
aims to create health organisations in which
there are mutually benecial outcomes by
having:
• Consumers as partners in planning, design,
delivery, measurement and evaluation of
systems and services.
• Patients as partners in their own care, to the
extent that they choose.
(6)
Eective partnerships exist when people are
treated with dignity and respect and provide
the foundation for compassionate delivery of
care and positive healthcare outcomes.
When patients need health care, they may
feel vulnerable, frightened, upset and
uncomfortable. Healthcare settings are
generally unfamiliar to the patient and
conversations that patients have with
healthcare clinicians, before, during and aer
care or treatment, can help reassure the patient
and allay some of their fears. The open sharing
of information helps strengthen patients’ trust
in the care team and improves the safety and
experience of patient care.
Person-centred care
Person-centred care is globally recognised
as the gold standard approach to safe, high
quality healthcare. It is a diverse and evolving
practice, encompassing concepts such as
patient engagement and patient empowerment.
Partnering with patients in their care is an
important pillar of person-centred care. It
focuses on the relationship between a patient
and a clinician, and recognises that trust, mutual
respect and sharing of knowledge are needed for
the best health outcomes.
When patients need to access the healthcare
system, they expect the care provided will be
safe and it will be sensitive to their needs and
wishes; the principles of person-centred care
are:
• treating patients with dignity and respect
• encouraging patient participation in
decision-making
• communicating with patients about their
clinical condition and treatment options.
Health services are required to incorporate
information on the diversity of of its consumers
and higher risk groups in to the planning
and delivery of care. (NSQHS action 1.15).
Partnering with Aboriginal and Torres Strait
Islander communities to meet their needs is also
referred to in (NSQHS action 2.13). Care should
be provided in a way that is respectful of, and
responsive to, cultural beliefs and practices,
whilst recognising the disparities faced by
Aboriginal and Torres Strait Islander peoples.
Immediate response or
unexpected situations
When things don’t go as expected, when
conditions change or when harm occurs, the
principles of safety and person-centred care are
even more important. The immediate action is
to ensure the patient is safe, and the necessary
care is provided, including the provision of
psychological support for the patient, sta and if
required, their family and or carer.
Whether the cause of the issue may be a
complication,error, an oversight, a safety
incident or a case of ‘we just don’t know right
now’, patients, families and carers need the
healthcare system to support them and commit
to nding out what happened and to making
improvements.
Handy tip
There are two information sheets available
to to support hospital and health services in
supporting the patient/family/carer when a
serious incident occurs:
Clinical incident management for Health
Service sta: Supporting the patient family
carer when a serious incident occurs
(Factsheet 2)
Patient/family/carer information sheet:
What you can expect when a serious
incident occurs

Patient/family/carer perspective | 15
Clinician disclosure
Compassion and an acknowledgment that
‘something unexpected has happened’ is
extremely important. Where the incident is
temporary or minor, the clinician most directly
involved in the incident or who rst recognises
the incident (medical ocer, nurse, midwife or
allied health professional) is usually the most
appropriate person to speak with the patient
and /or their support person.
The patient/ family/carer could be the rst
to see, feel or sense something isn’t right.
This can provide the healthcare team with
valuable information from the patient/family/
carer perspective; it is also an opportunity to
understand what they require. Not responding
or delaying disclosure creates more fear and
erodes trust. When any type of incident occurs,
patients need the healthcare clinician to meet
with them to:
• acknowledge the incident
• explain clearly what has happened in an
appropriate language style that all present
can understand
• sincerely apologise for any harm and
distress caused by the incident
• help the patient/family/carer understand
how and why it happened
• explain what will happen next and follow
through with commitments made.
Open disclosure
With incidents that result in unexpected death
or permanent harm, it is highly likely that further
discussion will be required, especially if there
are a number of facts that are unknown or if
additional treatment is required. The more
formal process is known as Open Disclosure
and should be oered to the patient or family or
carer. Open disclosure follows on from the initial
clinician disclosure. It should be delivered by a
senior clinician (an Open Disclosure Consultant)
who has had training in delivering information
in a clear, mpathetic and structured way. For
more detailed information, please refer to the
Open Disclosure Guide.
Handy tip
There are two types of disclosure used in
Queensland Health:
Clinician Disclosure is where the treating
clinician informs the patient of what has
occurred and provides an apology. This
disclosure should be the initial response to
all adverse events (SAC 1, 2, 3 and 4) and
occur as soon as practical aer the clinical
incident has been identied. For some SAC2
clinical incidents, along with SAC3 and
SAC4 clinical incidents, clinician disclosure
(the lower level of the two open disclosure
components) may be sucient.
(11)
Formal Open Disclosure is a structured
process ensuring clinical incidents are
addressed and responded to openly
between the patient, patient’s family
and/or carers, senior clinician and other
representatives of the Hospital and Health
Service. It is highly recommended that
formal open disclosure is oered for all SAC1
clinical incidents and may be necessary
for some SAC2 clinical incidents and
occasionally SAC3 and SAC4 incidents.
(11)
The analysis—what, how and why
it happened
In assisting the patient/family/carer to
understand what happened, it is highly likely
the senior clinician will be required to speak
to them as soon as possible, ideally within 24
hours and to also acknowledge that they may
be feeling a signicant level of grief or even
anger. It is highly recommended to actively seek
the patient/family/carer input and feedback
in the analysis process, enabling the patient/
family to contribute what they know from their
perspective.
(12)

16 | Best practice guide to clinical incident management Second edition - January 2023
Patients/families/carers will usually understand
that the circumstances around how and why
the event or incident happened may not be
fully known at the time of initial disclosure, and
that more information and time may be needed
to gather all the facts. The process needs to
be explained so that they can understand
what will happen next. This includes talking
to the patient/family/carer about the process,
including how the event or incident will be
analysed. It is important to allow plenty of
opportunity for the patient and/ or their family
/ carer to ask questions. They will also need
to be engaged in planning care following the
incident. It is best practice to invite them to
meet with a team member so that they can
provide their perspective and information
they know about the situation. In some cases,
the analysis process can be very simple and
straight forward. In other situations, it may be
more complicated and involve many dierent
people. Where possible, best practice would
involve the patient/family/carer from the
start of the process. An analysis of the facts,
particularly when serious harm is involved, is
not complete until all of the perspectives and
information from everyone involved, including
the patient/family/carer, have been gathered.
The analysis team may, at this point, consider
involving a consumer representative, who
is familiar with the perspective of patients,
families and carers, as part of the analysis
team: it is an important consideration, so the
family can be assured that their interests and
perspectives will be included.
(13)
Involving the patient/family/carer in the
analysis stage also demonstrates respect for
their point of view as the expert in their/their
family member’s experience. This emphasises
that the patient, not the system, is at the
centre of the concern. The goal is to make the
system safer for patients through fostering
understanding, learning and improvement.
While timely analysis is critical, there may
be a range of circumstances which may
prevent either the patient or a family member
participating in the analysis process straight
away. Try to be understanding and help nd
reasonable ways for them to participate. The
respect, empathy and understanding of what
they could be going through at the time, can
help rebuild their trust in clinicians and the
healthcare organisation.
Many patients/families/carers will want to keep
in contact with the organisation during the
analysis process. It is imperative that they are
provided with contact information and it may
help if a dedicated contact person is identied,
preferably someone with whom they already
feel comfortable.
In some situations where patients have been
seriously harmed or where there may be
signicant system failures, it may be dicult for
patients/families/carers (and sometimes even
the general public) to re-establish trust with the
healthcare organisation or system. Doubts may
arise that analysis teams, when recruited from
within the organisation, will not be as thorough
or unbiased as outside experts. In these
situations, consider the patient/family/carer
request for an external analysis team; noting
that ‘external’ may be a team from another
facility in the hospital and health service or a
clinical expert from outside the treating team.
Following the review of a serious incident, there
may be occasions where the patient, or their
family member is not satised with the process.
It is ideal if resolutions can be achieved at the
local level, however there may be times when a
referral to to Oce of the Health Ombudsman
(OHO) may provide an additional review
mechanism, as an independent body to assure
fair and transparent oversight in health service
complaint management. The OHO conducts
investigations into individual practitioners
where there may be evidence of professional
misconduct or where the practitioner poses a
serious risk to persons. OHO has the authority
to refer to the Australian Health Practitioner
Regulation Agency (AHPRA). The OHO can also
open an investigation into a health facility
or service to determine any systemic issues
aecting the quality of health services.
(14)
In more complicated situations, it may take
additional time to complete all aspects of the
analysis. Ensure that the patient and their
family/carer are aware of the timelines and keep
them informed of any delays or changes via the
nominated contact person.

Patient/family/carer perspective | 17
Following the analysis
Upon completion of the analysis, it is
recommended to meet with the patient/
family/carer in person if they wish, at a time
and place that is agreeable to them. Cultural
sensitivities should be taken into account when
planning how and where disclosure occurs and
consideration given to the inclusion of a local
indigenous liaison ocer/heath worker in the
meeting. If a date for follow-up was previously
agreed upon, keep to this commitment. If a
delay is expected, inform the patient/family/
carer, prior to the planned feedback date. Aim
to send the patient/family/carer information
or reports that will be discussed, in advance
of these meetings, so they can also analyse
them and come to the meetings prepared with
their questions. It is easier to communicate,
understand and re-establish trust when
everyone has the same information.
These meetings can be very emotional for
patients/family/carer members and for
clinicians. Ensure everything possible is
considered to make this time as easy as
possible for patients/families/carers. Ask
them about their perspective and include their
suggestions for learning and improvements.
The patient/family/carer view is a valuable
resource for nding eective solutions. Who
better to suggest improvement than those
who have experienced failures in care and
the system. Continue to talk with the patient/
family/carer about the next steps and how
they can continue to be informed or involved in
developing or promoting these improvements.
To the patient/family/carer this will show a
continuing commitment to their safety and the
safety of other patients. It also demonstrates
transparency.
It is essential that all analysis reports are
written, with the consideration that they can be
provided to a patient/family/carer, should they
wish to access the information. By providing a
written copy of the report, this can help them
come to terms with the consequences of the
incident and to also provide assurance that
everything is being considered to ensure this
doesn’t happen to anyone else.
A Root Cause Analysis (RCA) report with the
statutory protections in accordance with Part 6,
Division 2 of the HHB Act, can be provided with
the permission of the commissioning authority,
to any person they believe has sucient
personal or professional interest in the incident
(s.115 Hospital and Health Boards Act 2011).
Partners in building trusting
relationships
At a broader level, patients want to know that
all current best practices related to national
guidelines are being used in your Hospital
and Health Services, and that patient safety
incidents that do occur are analysed, actioned
and implemented, and the learnings from these
incidents are shared to prevent recurrence.
As new and improved ways are considered
and used to incorporate safety and quality into
healthcare, seek to involve patients, families
and carers in the process. Partnering with
consumer representatives, patients, families
and carers assists to ensure that these advisory
experiences are benecial for all parties.
(14,15)
Implementing systems to support partnering
with patients, carers and other consumers
to improve the safety and quality of care is a
requirement of meeting NSQHS Standards.
By enabling skilled and experienced consumer
representatives to be involved in adverse
event reviews, a strong patient-focused
perspective can result with a number of
benets for the health organisation: including
being involved in interviewing patients,
ensuring the voice of patients/families/carers
is heard and advocating for patient-centred
recommendations.
(13)
Partnering with Consumers Standard

Close
the
loop
Follow
through
Analysis
process
Prepare
for
analysis
Immediate
response
Before
the
incident
1
2
3
4
5
6
Clinical Incident
Managment
Process

Close
the
loop
Follow
through
Analysis
process
Prepare
for
analysis
Immediate
response
Before
the
incident
1
2
3
4
5
6
Clinical incident management
Clinical incident analysis cannot be addressed in isolation from the multitude of activities that take
place following a clinical incident. While there will be some variation in how healthcare organisations
manage clinical incidents, the basic steps will be consistent. There is interconnectivity and
interdependence between the identied activities, noting some may take place simultaneously. Figure
2 shows how incident analysis is an integral part of the incident management process and will be used
throughout the Guide.
Figure 2. Clinical Incident Management Process Steps
Close
the
loop
Follow
through
Analysis
process
Prepare
for
analysis
Immediate
response
Before
the
incident
Share what was
learned (internally
and externally).
Implement
recommended
actions.
Monitor and assess
the effectiveness
of actions.
Understand what
happened.
Determine how and
why it happened.
Develop and manage
recommended actions.
Key principles.
Ensure leadership
support. Create a safe
and just culture.
Key concepts.
Care for and support
patient/family/clinicians
/others. Report incident.
Secure items. Begin
disclosure process.
Reduce risk of imminent
recurrence.
Preliminary assessment.
Select an analysis
method.
Identify the team.
Coordinate meetings.
Plan for/conduct
interviews.
Depending on the nature of the incident,
these activities may be performed by a few
individuals or a larger team. Refer to Appendix
A (Analysis team membership and roles and
responsibilities) for further information. In
some cases, there may be dierent teams
engaged at at each of the stages of the incident
management cycle (e.g. there may be dierent
teams/members who conduct disclosure
processes to those who conduct analysis and
review processes and those who manage
implementation processes).
The Commission’s Incident Management Guide
outlines seven key principles of eective clinical
incident management:
1. Transparency
2. Accountability
3. Partnering with consumers
4. Open, fair and just culture
5. Act in a timely way
6. Prioritisation of action
7. Shared learning.
The investigation and review
methodology
There are a number of dierent methods
available to investigate a clinical incident.
It has been acknowledged that healthcare
is more complex compared to aviation and
other high-risk industries given the dynamic
nature of the interactions between multiple
clinicians, vulnerable patients, and complex
care processes. Queensland Health does not
stipulate to hospital and health services what
type of method/analysis must be undertaken
to investigate a clinical incident. This decision
remains at the discretion of the of the hospital
or health service in terms of what type of
review methodology is best suited for the
type of incident. This Guide focuses on three
approaches - Comprehensive, Concise and
Multi-incident analyses.
Clinical Incident Management Process | 19

20 | Best practice guide to clinical incident management Second edition - January 2023
System based analysis review
versus accountability review
In most organisations, there are two types of
formal reviews that are generally available to
manage unexpected clinical outcomes and
clinical incidents:
• System-based analysis reviews -
Comprehensive, Concise and Multi-analysis
for system improvement
• Accountability review
This Guide is predominantly focused on system
improvement, whereas accountability reviews
are directed to individual performance or
behaviour and are managed by the appropriate
health practitioner stream i.e., Medical,
Nursing and Midwifery, Allied Health or others
(or through an administrative process e.g., a
Health Service Investigation under the HHB Act).
Further advice, including legal advice may be
needed for an accountability review, depending
on the particular circumstances. However, it
is important that all parties understand that
poor individual performance may occur due to
ineective systems e.g., inadequate training,
ineective policy and/or guidelines.
It is important to protect the integrity of the
systems-based incident analysis process
from a situation where there is potential for
administrative, disciplinary, or criminal action.
If reasonable concerns about individual
performance or conduct arise during the course
of a system-based analysis review, this should
be escalated, and an appropriate accountability
review set up as a separate process to
address the identied issues. Likewise, issues
concerning system issues or failures raised
during an accountability review should be
referred to a system improvement review.
In some circumstances it may be possible
and appropriate to run parallel systems and
accountability reviews. However, it is imperative
that information not be shared from one
process to the other and that all participants
are aware of the distinction between the two.
This is to ensure the integrity of each review
and that any legal duty of condentially is not
breached. When the parallel investigations are
complete, the learnings generated from each
process can be valuable for improvement. In
these situations, HHSs are advised to seek local
human resource and legal advice.
Investigation legislation
Root cause analysis
A review team may determine that a root cause
analysis (RCA) (refer to Part 6, Division 2 of
the HHB Act), is the preferred methodology
to review an incident. If during the RCA, the
team becomes aware of ‘a blameworthy act’
or the capacity of a person directly involved
in providing the health service was impaired
by alcohol consumed, or a drug taken by the
person; or a member of the team reasonably
believes the event involves behaviour of a
registered health practitioner that constitutes
public risk notiable conduct, then the RCA
must stop and the commissioning authority
for the RCA must be notied. The Health
Ombudsman must also be notied about the
conduct (refer to section 102-103).
The HHB Act provides a number of
mechanisms for consideration beyond an RCA,
including clinical reviews and health service
investigations. The following is a brief overview.
Clinical review
The function of a clinical review (refer to Part 6,
Division 3 of the HHB Act) is to conduct a clinical
review and to provide expert clinical advice.
The clinical reviewer may make
recommendations on ways in which the safety
and quality of public sector health services can
be maintained and improved. A clinical reviewer
can be appointed to provide clinical advice to:
• the Department Chief Executive or a HHS
Chief Executive
• a person/entity whose role includes
improving the safety and quality of public
sector health services; or
• a health service investigation (HSI).
A clinical reviewer must prepare and provide a
report to the appointer for each clinical review.
Statutory protections limiting further disclosure
apply to a clinical review report other than, as a
result of a review undertaken to provide clinical
advice to an HSI. The purpose of these clinical
review reports is for improvement in clinical
services and reports are not accessible under
an order or admissible in any proceedings.
A clinical review report prepared to provide
clinical advice to a HSI however, may be
admissible in civil, criminal and disciplinary
proceedings.

If during a clinical review (except one
undertaken to provide clinical advice to a HSI),
a clinical reviewer reasonably believes that a
matter under review involves a blameworthy
act (refer to Part 6, Division 2, Section 94 of the
HHB Act), the clinical reviewer must:
• stop the review
• give written notice to the appointer that
states the review has been stopped and the
reasons that the clinical reviewer formed the
reasonable belief.
Health service investigations (HSI)
The function of an health service investigation
(HSI) (refer to Part 9 of the HHB Act) is to
investigate and report on any matters relating
to the management, administration or delivery
of public sector health services, including
employment matters. A clinical reviewer may be
appointed to provide clinical advice to an HSI.
A health service investigator must prepare and
provide a report to the appointer for the HSI.
Where a clinical reviewer is advising, the HSI
must:
• have regard to any report provided by the
clinical reviewer; and
• attach the reviewer report to the
investigation report.
An HSI report (and any attached clinical review
report) may be admissible in civil, criminal and
disciplinary proceedings or by other legal order.
The appointer must be satised the clinical
reviewer or health service investigator is, among
other things, qualied for the appointment
because they have the necessary skills,
knowledge and experience or expertise. Any
such appointment is then set out in writing (the
instrument of appointment). In these situations,
it is recommended that legal advice is sought.
Duty of Condentiality
A statutory duty of condentiality under the HHB
Act applies in the performance of RCA, clinical
reviews and HSI, with requirements for not
disclosing information provided to them in that
capacity, except in circumstances prescribed
under the legislation. Refer to Appendix B
Incident reporting and investigation legislation
for further information.
Clinical Incident Management Process | 21

22 | Best practice guide to clinical incident management Second edition - January 2023
Key principles
The following principles in Table 1 form the foundation for eective clinical incident management.
The employ of these principles will assist with reducing the risk of recurrence of similar patient safety
incidents and aim to result in improved healthcare outcomes. Sta in Queensland Health’s hospitals
and health services are encouraged to support, enact and openly communicate these principles as
part of a culture of patient safety, disclosure, quality and learning from incidents.
Table 1. Clinical Incident Management Principles
(16)
Principle Description
Transparency
Health services should provide the patient, family or carer, and sta with an
honest, open and full explanation of what happened, why it happened and what
actions have or will be taken, as per Queensland Health’s Open Disclosure Guide,
2020.
Accountability
Health services have a duty to take reasonable care to avoid harm to patient, family
or carer, and sta.
Partnering with
consumers
The patient, family or carer who are associated with the incident are asked to
contribute to the clinical incident management process as appropriate, during the
investigation and review.
Health services should seek to support the participation of a patient/consumer
representative in reviewing serious clinical incidents.
Open, fair just culture
Health services should create a patient safety culture of trust, fairness, learning
and accountability that encourages sta, patients, families or carers to feel safe to
speak up when an incident occurs and to report incidents. The workforce is fairly
supported when the system fails, and errors occur.
Act in a timely way
Health services take action to correct problems in a timely manner with clear
allocation of responsibility.
Prioritisation of action
Health services prioritise actions that will have a high impact on harm prevention in
areas of high risk and where there is high achievability of improvement.
Shared learning
Health services share the learnings from clinical incidents to prevent further similar
patient harm occurring.
– Key principles
– Ensure leadership support
– Create a safe and just culture
– Key concepts
Step 1: Before the incident

Step 1 – Before the incident | 23
Ensure leadership support
Building high-reliability health organisations
and systems for a strong patient safety culture
that protect patients daily from harm, requires
strong leadership at all levels.
(17)
Leaders must
commit to creating and maintaining a culture of
safety as inadequate leadership can contribute
to adverse events. An engaged and skilled
leadership team is paramount to improving
patient safety. Having board members who
are skilled in quality and safety has played a
positive role in influencing safety.
The Australian Commission on Safety and
Quality in Health Care (the Commission)
Clinical Governance Standard aims to ensure
organisations have systems in place to maintain
and improve the reliability, safety and quality
of health care. This Standard recognises the
importance of governance, leadership, culture,
patient safety systems, clinical performance and
the patient care environment in delivering high
quality care.
(6)
Clinical incident management requires a whole
of organisation approach that should foster
a just culture and incorporate leadership
responsibilities at each organisation level,
including Board Directors and Executive.
Hospital and health services board directors
and executives have a key role in cultivating
a culture that leads to improved patient care,
by the establishment of specic committees
overseeing all safety and quality activities
across the organisation and the systems.
Leadership prominence in clinical departments
allows for frank discussions around safety
concerns and can impact positively on safety
culture when issues are raised, discussed and
solutions identied and implemented. The
reaction of leaders to an adverse event is crucial
in determining if the heath service learns from
the incident or not, and hence, if future harm to
patients is reduced.
Pressure to act can mount quickly when a
patient experiences an incident. Organisations
can best handle the situation if they develop
a plan ahead of an incident occurring that
describes the steps and responsibilities for
various actions (who is doing what, how and
when) and indicates the resources available
(policies, procedures, checklists, skills) to
manage the incident.
The incident management plan requires
visible leadership support at all levels of the
organisation and is reinforced by a safe and just
culture in place ahead of the incident.
(18)
Plans
and procedures need to be tested, updated
and revised periodically to ensure they are
aligned with the evolving culture, structure and
processes of the organisation.
Organisations that continuously build and
maintain resilience in their structures, functions
and way of thinking about clinical incidents are
better prepared to manage the unexpected.
Five attributes characterise these organisations:
1. Preoccupation with failure—to avoid failure
we must look for it and be sensitive to early
signs of failure.
2. Reluctance to simplify—to understand the
more complete and nuanced picture of an
incident avoids over-simplication, labelling
and clichés.
3. Sensitivity to operations—systems are not
static and linear, but rather dynamic and
nonlinear in nature. As a result, it becomes
dicult to know how one area of the
organisation’s operations will act compared
to another part.
4. Commitment to resilience—the organisation
must maintain its functions during high
demand events. Resilience has three
components:
• absorb strain and preserve function,
despite adversity
• maintain the ability to return to service
from untoward events
• learn and grow from previous episodes.
5. Deference to expertise—this may include
deference downward to lower ranking
members of the organisation, with greater
emphasis on an assembly of knowledge,
experience, learning and intuition rather
than on one’s position in the organisation.
Credibility, a necessary component of
expertise, is the mutual recognition of skill
levels and legitimacy.
(19,20)
To build and support both resilience and
responsiveness in plans, organisations are
encouraged to tap into the learning generated
from previous incidents (near misses are
of great value),
(21)
improvement eorts and
learning from multi-incident analyses.

24 | Best practice guide to clinical incident management Second edition - January 2023
The importance of a strong patient
safety culture
The patient safety culture of an organisation is
a major component of supporting safety and
quality improvement. Healthcare organisations
are required to build and maintain a safety culture
and this is well articulated through a range of the
Commission’s work. Safety culture is dened by
the Commission as:
‘the product of individual and group
values, attitudes, competencies and
patterns of behaviours that determine
commitment to and the style and
prociency of an organisation’s health
and safety programs’.
(4)
Positive safety cultures in health care are
demonstrated by strong leadership, which aims to
drive and prioritise the safety of all. Commitment
from leadership and management personnel in
this context is important because their actions
and attitudes influence the perceptions, attitudes
and behaviours of members of the workforce
throughout the organisation.
(4)
Organisations with positive safety cultures have:
• strong leadership to drive the safety culture
• strong management commitment, with safety
culture a key organisational priority
• a workforce that is engaged and always aware
that things can go wrong
• acknowledgement at all levels that mistakes
occur
• non-blame, non-punitive response to error
• ability to recognise, respond to, give feedback
about, and learn from, adverse events.
(4)
A positive safety culture is comprised of many
things, including openness, honesty, fairness
and accountability. It requires strong leadership
approaches that build and drive safety by
encouraging the reporting of incidents and
safety hazards. It supports opportunities for
safety training and preparedness. It promotes
understanding, learning and improvement. It
requires flexibility and resilience, so that people,
unexpected situations and priorities can be
managed in a timely and eective manner.
(22)
It promotes person-centred care and partnering
with consumers.
The World Health Organisation (WHO) states:
‘all reporting and learning systems,
whether large or small scale, must create
rst a positive culture in which reports
are encouraged and valued, and sta are
praised for participating.’
(23)
All sta are responsible for identifying and reporting
incidents. Most incidents are identied at the time;
however, some may be identied later aer the
event. Sources of identication can be through
complaints, media, audits, morbidity and mortality
committee meetings, safety committees and through
general discussion. If incidents are NOT reported,
learnings cannot be made and there is a high chance
of a recurrence.
Occasionally, clinicians will indicate that there is no
need to analyse an incident because they believe
that the harm resulted from a known complication.
It is important to understand that with advances in
care some complications will, over time, become
preventable and, therefore, classied as patient
safety incidents. Furthermore, clinical incidents
that are coupled with complications and without
conducting an incident analysis, opportunities for
learning and improvement may be lost.
An organisation with a blame culture,
is detrimental to patient safety and
creates stressors on sta who may feel
undervalued, unable to intervene to
improve safety and most importantly likely
to avoid reporting or involvement for fear of
repercussions. A fear of blame is a principle
reason for not reporting incidents.
The incident analysis process is most eective when
it is conducted within a mature safety culture that has
been established and underpinned by a restorative
just culture. These types of cultures are largely
based on an organisation ‘possessing a collective
understanding of where the line should be drawn
between blameless and blameworthy actions.’
(2)
Dierences are drawn between actions of intent,
recklessness and those of unforeseen circumstances
or complications of care.
Culture cannot be implemented solely based
on policy or procedure; rather, it needs to be
consistently fostered over time and by example, at
all levels in the organisation. Ultimately, everyone
in the organisation has a role in helping to build
and maintain a safety culture.

Step 1 – Before the incident | 25
Applying a Restorative Just Culture
(RJC)
Moving from a just culture to create a
restorative just culture is an approach that
replaces the backward-looking accountability,
with an aim to repair trust and relationships
damaged or hurt aer an incident. A RJC
creates a healing, learning and improving
approach.
(29)
It has been dened as “a process
where all stakeholders aected by the injustice
have an opportunity to discuss how they have
been aected by the injustice and to decide
what should be done to repair the harm”.
It
asks three questions:
• Who has been hurt?
• What are their needs?
• Who should meet those needs?
(30)
The process emphasises the importance of
participation by those who have a direct stake
in the event to tell their story; this is a powerful
way to share their experience with others, to
empower them and be involved in review process.
Acknowledging who is hurt and what their needs
are, is the rst step towards becoming truly ‘just’.
The following summary provides a comparison
between a Just Culture and a Restorative Just
Culture.
Just Culture Restorative Just
Culture
Which rule was
broken?
Who is impacted?
How bad was that
breach?
What do they need?
What should the
consequences be?
Whose obligation is it
to meet that need?
Accountability Accountability
Account is settled,
paid
Account is told, honest
story
Backward-looking
accountability
Forward-looking
accountability
Who is responsible? What is responsible?
A just culture approach
A just culture is a culture of trust, fairness,
learning, and accountability that asks the question
“what went wrong?” not “who caused it?”.
(24)
A
Just culture has its origin in the aviation industry
in 1980s, when the rst fully developed theory of
a just culture was published in James Reason’s
1997 book, Managing the Risks of Organizational
Accidents.
(25)
This concept was then applied by
David Marx into the healthcare setting in 2001.
(26)
In contrast to a blame culture, a just culture aims to
create an atmosphere of trust and encourages the
reporting of incidents and hazards by all, to help
the organisation learn from the incident. Honest
mistakes are seen as learning opportunities for the
organisation and the employees.
(27)
The just culture concept is related to systems
thinking which purports that incidents/accidents
are generally the result of system errors rather than
an individual directly involved or responsible. It
recognises that individual clinicians should not be
held accountable for system failures, over which
they have no control; however, it holds individuals
accountable for willful misconduct or gross
negligence.
(24,25)
Whilst investigators principally attempt to
understand why system failings occurred, many
just culture models worked to rstly consider
what went wrong in retributive terms, by asking
questions of human behaviour (errors/slips, at risk
behaviour or recklessness); a backward looking
accountability.
(24)
Evidence of patient safety has
shown that individual acts are responsible for only
a very small minority of the incidents that occur.
(28)
Handy tip
The United Kingdom, National Health Service
(NHS) has incorporated the Clinical Incident
Decision Tree into the Just Culture Guide. This
guide moves away from asking ‘Who was to
blame?’ to asking, ‘Why did the individual act
in this way?’ when things went wrong.
It should help health service managers
and senior clinicians decide what, if any,
management actions are required for sta
involved in a serious patient safety incident
and promote fair and consistent treatment of
sta within and between health services.
The NHS Guide has been adapted for use in
Queensland Health as A just culture approach.
Refer to Appendix C (A just culture approach)

26 | Best practice guide to clinical incident management Second edition - January 2023
What are the goals of a
restorative just culture?
Meeting all the following goals is essential to
supporting a just culture:
• moral engagement – all parties are engaged
in considering the right thing to do now
• emotional healing – helps cope with feelings
of guilt, humiliation; oers empathy
• reintegrating practitioner – does what is
needed to get person back into their job
• organisational learning – explores and
addresses systemic causes of harm.
(30)
Access to: Sidney Dekker’s Restorative Just
Culture checklist
The restorative just culture framework (Appendix
D) identies as part of a restorative just culture
framework, the various groups that can be hurt
following an incident; the rst and second
victims, the organisation and the community.
Table 2. Safety I and Safety II concepts
Safety I Safety II
Denition of safety That as few things as possible go wrong.
That as many things as possible go
right.
Safety management
principle
Reactive, respond when something
happens or is categorised as an
unacceptable risk.
Proactive, continuously trying to
anticipate developments and events.
View of the human
factor in safety
management
Humans are predominantly seen as a
liability or hazard. They are a problem to
be xed
Humans are seen as a resource
necessary for system flexibility and
resilience. They provide flexible
solutions to many potential problems.
Accident investigation
Accidents are caused by failures and
malfunctions. The purpose of an
investigation is to identify the causes.
Things basically happen in the same
way, regardless of the outcome. The
purpose of an investigation is to
understand how things usually go right
as a basis for explaining how things
occasionally go wrong.
Risk assessment
Accidents are caused by failures and
malfunctions. The purpose of an
investigation is to identify causes and
contributory factors.
To understand the conditions where
performance variability can become
dicult or impossible to monitor and
control.
Key concepts
This Guide directs our thinking beyond the
linear representation of patient safety incident
analysis of Safety I, by emphasising concepts
related to Safety II, systems thinking, human
factors and complexity, within the dierent
system levels. It is important to understand the
dierence between simple, complicated and
complex systems for a deeper awareness of how
clinical incidents occur in healthcare to enable
the development of improvement strategies.
Safety I and Safety II
The Safety I approach places clinical incidents
as the focus point and aims to identify the
causes of adverse events, while Safety II aims
to understand how things usually go right
and this forms the basis for explaining how
things go wrong. Safety II approaches aim to
complement, not replace Safety I. It assumes
that the system’s ability to respond and adapt
to varying conditions, allows for things going
right. Looking at the work environment and
Work-As-Done rather than Work-As-Imagined,
shows the flexibility and adaptiveness of people
within the system; this variability is necessary
for the system to function; human variability.
(31)
Table 2 shows the dierence between the
Safety I and Safety II concepts.
(8)

Step 1 – Before the incident | 27
System
A system is an interconnected set of elements
that is coherently organised in a way that
achieves something; it consists of elements,
interconnections and a function or purpose.
(32)
Systems can be generally classied in two
categories: mechanical (e.g. cars, planes) or
adaptive (e.g. organisms or organisations).
Mechanical systems have a high degree
of predictability and are easier to control
because they respond consistently to the
same stimulus. Adaptive systems have a low
degree of predictability because all parts of
the system do not respond in the same way to
the same stimulus. When adaptive systems
are also complex, there is an additional
factor that decreases predictability—one
individual’s actions can change the context for
other individuals working within the system.
(33)
This can be either helpful or harmful. It
can be helpful because dierent responses
and changes in context generate innovative
approaches and better solutions. It can also be
harmful because this unpredictability increases
variation and thus the potential for harm.
Health care is a complex adaptive system due
to the large number of interacting services and
is adaptive in that the system is able to self-
organise and learn.
(34)
System thinking and human factors
(ergonomics)
At its core, the science of human factors
examines how humans interact with the world
around them. It can help determine how and
why things go wrong. Human factors science
draws upon applied research in many areas,
such as biomechanics, kinesiology, physiology
and cognitive science, to dene the parameters
and constraints that influence human
performance. This specialised knowledge
is used to design ecient, human-centred
processes to improve reliability and safety.
Because systems-thinking and human factors
impacts all levels of patient safety incident
management, these concepts have been
integrated throughout the Guide in addition to a
brief overview here.
Historically, when an incident occurred, the
tendency was to look for the most obvious
explanation of what and why it happened.
In most cases, individual human error was
identied as the cause, primarily because it
was easy to identify and appeared to be easy
to x.
(35)
This approach ignored the underlying
contributing factors that led to the incident
and thus presented a shallow analysis of the
circumstances. The outcome of such an analysis
may have included the creation of new policies/
procedures, additional training, disciplinary
actions and/or an expectation of increased
personal vigilance. The focus was almost
exclusively directed at improving individual
performance and as a result, this approach was
likely unsuccessful in preventing the same or
similar incident from occurring again.
Patient safety experts now strongly advocate
for a way of thinking that views human error as
a symptom of broader issues within a poorly
designed system, such as an adverse physical
or organisational environment. Dekker
(36)
refers
to an old and new view of human error. In the
old view, the objective is to nd the individual’s
inaccurate assessments, wrong decisions and
bad judgement. In the new view, the objective
is not to nd where the person went wrong, but
instead assess the individual’s actions within
the context of the circumstances at the time. A
deeper inquiry into the circumstances will yield
system-based contributing factors.
Finding contributing factors that are embedded
in flawed systems requires targeted strategies.
Knowledge of the human factors involved
is both useful and important when asking
questions during the incident analysis process
and can help the analysis team focus on
issues related to systems and not on individual
performance. An eective incident analysis
always incorporates human factors.
Human factors or ergonomics are scientic
disciplines concerned with: “the understanding
of the interactions among humans and other
elements of a system, and the profession that
applies theoretical principles, data and methods
to design in order to optimize human well-being
and overall system performance”.
(37)

28 | Best practice guide to clinical incident management Second edition - January 2023
Taking a human factors approach means that
when safety incidents occur, it is important to
have a non-punitive culture. Instead of blaming
individuals for events, the systems approach
focuses on:
• building systems to reduce potential risks
and prevent future errors
• building system defences to reduce the
likelihood of errors resulting in patient harm.
The overall human factors philosophy is that the
system should be designed to support the work
of people, rather than designing systems to
which people must adapt.
(38)
Because systems-thinking and human factors
impacts all levels of patient safety incident
management, these concepts have been
integrated throughout this Guide and depict
a non-linear approach that includes the
consideration of ‘categories of factors types’
such as:
• patient factors
• task factors including technology
• team factors
• individual (sta) factors
• work environment factors (equipment,
devices)
• organisational and management factors
• external factors (Government, National,
International, economic and regulatory,
other organisations).
Complexity
Complexity refers to the density of interactions
between dierent components in a system
and which produce roles and behaviours that
emerge from those interactions.
(39)
Complexity
science suggests that errors, threats to safety
and accidents are not caused by any one thing,
as in a conventional Root Cause Analysis, but
emerge from the non-linear interactions of all
components.
(40)
Complex systems are characterised by features
that may operate in patterned ways, but
the interactions within them are continually
changing. With complex systems, there is
a low level of agreement on the outcomes
or processes because situations involve
multiple individuals or processes and there is
a high degree of heterogeneity among them
(e.g. dierent departments are involved). In
addition, teams may self-organise around
areas of competence, making relationships
and resulting interactions even more fluid. The
process for transferring a critically ill patient
between facilities or an error with a blood
transfusion involving labelling, in a pressurised
environment with electronic systems, would be
an example of a complex system.
Complicated systems have many moving parts
or tasks in a process, there are many possible
interactions, but they operate in a patterned
way. It is possible to make accurate predictions
about how a complicated system will behave.
They generally involve several individuals,
oen from dierent professions. The patient
admission process would be an example of a
complicated system.
Simple systems contain few interactions and
are extremely predictable. The same action
produces the same result every time. There is
also a high degree of agreement on outcomes
and processes. The process for obtaining a
blood sample via venipuncture would be an
example of a simple system.
Handy tip
In clinical incident analysis, complexity
should be considered when selecting
an incident analysis method, analysing
contributing factors and building
recommendations. The degree of
interconnectedness and the relationships
between the dierent parts of the system
also help to dierentiate complicated
and complex scenarios. In a complicated
scenario the relationships can be simulated
and claried (which increases the
predictability), while in a complex system
or situation this is not possible because the
elements are not stable—they interact and
influence each other continuously (making
predictability impossible). See Figure 3
matrix for considering the distinctions.

Step 1 – Before the incident | 29
Figure 3. Complex, complicated, simple
systems matrix
(39)
Relatively
complex
Simple
Complex
Complicated
Number of components
Degree of interrelatedness
Close to Far from
ManyFew
Sphere of influence
Sphere of influence refers to the number and
strength of interconnections between the parts
of the system.
(41)
A particular contributing factor
could be influenced by any number of other
factors. For instance, an incident may result
from the failure to safely transfer a patient
from a bed to a wheelchair. One contributing
factor may be that the hoist used to facilitate
the patient transfer is new to the service area.
Another contributing factor may be that training
did not occur before the hoist was put into
operation. In this case, the lack of training and
the new hoist influenced one another.
Additional contributing factors may be the
unavailability of a trainer from the supplier and
that the hoist was moved into service sooner
than planned to replace another unserviceable
hoist device. All of these factors (new hoist, no
training, no training available from the supplier
and the urgent replacement of an unserviceable
hoist) when taken together, create a confluence
of factors that acted upon one another and
contributed to the incident.
In clinical incident analysis, the
sphere of influence should be
considered when analysing and
prioritising contributing factors,
especially when using the
constellation diagram.
The concept of sphere of influence is
demonstrated in the analysis of incidents
with the use of a constellation diagram. The
constellation diagram helps those responsible
for analysis to visualise the incident and factors
that contributed to the incident (explained in
detail in Appendix E Creating a constellation
diagram). The sphere of influence is visualised
by connecting the contributing factors that
influence one another. It is not intended to
be linear in its representation. This step will
support understanding of how a particular
grouping of contributing factors, acting upon
or in connection with one another, combined
to produce a specic incident that may prove
problematic for other patients in similar
circumstances if not addressed.
In a complex incident, where elements
constantly interact and influence each other, the
constellation diagram and contributing factors
identied should be considered a snapshot
of the incident and the context. The role of the
analysis team is to develop recommended
actions to address the identied local factors.
Based on this snapshot view, decision makers
and leaders of the organisation need to identify
and act on ndings that aect the organisation
as a whole.
System levels
Systems are generally viewed from various
levels (stratication) because there are
dierences in goals, structures and ways of
working in dierent parts of the organisation.
There is general agreement that the following
four levels (three internal and one external to
the organisation) are representative of most
systems.
(42)
See Figure 4.
• Micro—the point where the care clinicians
interact with the patient (e.g. clinical team or
service area that provides care).
• Meso—the level responsible for service
areas/clinical programs providing care for
a similar group of patients, typically part of
a larger organisation (e.g. a home care or a
cardiac care program).
• Macro—the highest (strategic) level of
the system, an umbrella including all
intersecting areas, departments, clinicians
and sta (e.g. boards, healthcare network,
integrated health system or region that
includes several organisations).
• Mega (external)—the level outside the
organisational boundaries that influences

30 | Best practice guide to clinical incident management Second edition - January 2023
the behaviour or more than one system.
The dierent sectors of healthcare such as
regulatory bodies, licensing organisations,
professional groups, liability protection
providers, state and federal governments,
national patient safety and quality
organisations, the healthcare industry and
the community all fall into this category.
Figure 4. System levels
Patient
Family/carer
Micro
Individual/provider
Meso
Service area
Macro
System
Mega
External system
Handy tip
In analysis, system levels should be
considered when selecting the method of
analysis, analysing contributing factors,
or prioritising recommended actions. It is
important to maintain focus on the level
where activities will predominately take
place and how that level is connected with
(or influences) the neighbouring levels.
Context
Context is dened as the interrelated conditions
in which something exists or occurs—
environment setting.
(43)
Context can include a
combination of relevant internal and external
conditions
(44)
specic to the incident and system
that influence the incident analysis process.
When conducting the analysis or managing the
incident, teams need to consider internal factors,
such as pressures and priorities generated from
any of the following:
• incident data (historical reports or
recommendations/actions) from the internal
reporting system, patient complaints,
accreditation reports, insurance claims,
civil litigation, etc
• short and long-term strategic priorities and
action plans
• resources available (human and nancial),
including leadership support and
coordination.
External pressures such as the following also
require consideration:
• regulations, requirements, preferred practices
• evidence from literature (e.g. the risk and
frequency of the incident, its impact and cost,
evidence-based interventions)
• information from public patient safety reports/
databases including Queensland Health
Patient Safety Notications: https://qheps.
health.qld.gov.au/psu/alerts/alerts
• anticipated demands from patients, public,
media and other stakeholders.
In clinical incident analysis, context
should be considered when selecting
a method of analysis, analysing
contributing factors and prioritising
recommended actions.
Without a good understanding of the context,
clinical incident analysis may not have the
desired impact because the recommendations
generated are not craed to t the reality of
the organisation. To accurately understand
the context, the involvement of organisational
leadership is essential.

Step 2 – Immediate response | 31
Care for and
support patient/family/
clinicians/others.
Report incident.
Secure items.
Begin disclosure process.
Reduce risk of imminent
recurrence.
Step 2: Immediate response
Care for and support of patient,
family, carer, clinicians and others
A clinical incident can be a very traumatic
experience for the patient, their families or carers
and clinicians involved. Generally, the rst action
aer recognising that an incident has occurred is
to care for and support the patient and the family,
as well as ensuring the safety of other patients
who may be at risk.
Attending to the safety and wellbeing of the
clinician/s involved and others (second victims)
is also a necessity.
(2)
Incidents have the capacity
to have lasting eects on all those involved
including the organisation. It is essential that the
hurts, needs and obligations are discussed with
all parties.
(30)
In a restorative just culture, when
a serious adverse incident occurs, it is expected
the senior leaders would attend the scene
immediately for support.
Report incident
While each situation will be dierent and guided
by individual organisation policies and practices,
the next activity aer providing support to
those involved generally includes reporting the
incident so that further steps can be taken to
manage the incident. All sta are responsible
for identifying and reporting incidents in a non-
punitive environment. In Queensland Health,
this involves recording the details of the incident
in the incident management system, RiskMan.
Incidents with a high potential for harm to the
patient, sta or reputation of the organisation
should also reported verbally as part of the
immediate response. See the Immediate response
checklist and action plan. Timely reporting assists
in understanding the next steps, such as whether
further analysis is needed, and/or whether
additional resources and other actions, such as
further notications are required. The appropriate
manager or other recipient of the report will, at a
minimum, analyse the facts of the incident and
gather any additional information to ensure a
preliminary understanding of what happened.
Any contributing factors identiable at this point
will also be documented.
Reporting is the trigger for a chain of internal
notications that, depending on the nature
of the incident, will target individuals and/or
units at dierent levels of the organisation (e.g.
attending chief executive ocer, risk management
committee, medical managers, health record
sta, unit or program managers, public relations).
External notications may also be required to
ensure alignment with regulations and to maintain
the organisation’s reputation as per legislation,
policy, protocols (e.g. State Coroner, Department
of Health) and current context (e.g. media).
Eective, timely and respectful internal and
external communication can result in increased
trust of all stakeholders, including the public. It is
recommended that organisations develop internal
guidelines for this purpose. For serious adverse
incidents likely to have an impact on safety,
services, sta, reputation or resources, a Hot Issue
Brief (HIB) to the Department of Health maybe
required.
– Care for and support patient,
family, clinicians, & others
– Report incident
– Secure items
– Begin disclosure process
– Reduce risk of imminent
recurrence

32 | Best practice guide to clinical incident management Second edition - January 2023
Secure items
Consider if any items/equipment related to the
event need to be secured for testing and for
analysis by the analysis team. Items include,
but are not limited to, biomedical equipment, IV
solutions, medications, packaging, garments,
etc. The items should be carefully labelled
(including lot numbers and serial numbers in
the event of a product recall or if further testing
is needed) and placed in a designated location
(or given to a designated person) where they
are protected, secured and access is restricted.
Photographs of the items and workspace may
also prove helpful. Health records in whatever
format also need to be secured and access
to them should be controlled. If the patient is
receiving ongoing care, sta will need to have
access, including to paper charts, that will
need to remain with the ward or unit chart if the
patient is receiving ongoing care.
Handy tip
During an analysis of an incident, it
is helpful to gather materials such as
equipment and any other materials used
during or close to the time of the incident.
Essentially, you need to review anything
that may have influenced the human-system
interaction during the incident and therefore
may constitute a possible contributing
factor. For instance, when reviewing a
medication error, you would seek to make
the following items available for further
examination:
• medication administration record
• prescribers’ order form
• medication vial or syringe labels
• IV pump
• other medical equipment used to deliver
the medication.
You will need to consider the values that
were written or entered into the medication
record. You may also need to consider the
design of the materials or equipment to see
if they may have been a source of confusion.
Also, it may be helpful to analyse the
organisational chart, shi schedules, room
or floor layout and measurements of the
work environment, including room lighting
or noise level.
Begin the disclosure process
Senior clinicians from the organisation should
begin the disclosure process with the patient,
family or carer as soon as possible aer the
incident (within 24 hours). Empathic and
timely disclosure can help patients, family and
sta deal with the consequences of a clinical
incident. Throughout the disclosure process, due
consideration must be given under Queensland’s
Human Rights Act 2019 to undertake public
functions in a principled way that places
individuals at the centre of decision making and
service delivery, ensuring that all have their human
rights respected, protected and promoted.
Disclosure may be a one o events or is an ongoing
process in which multiple disclosure conversations
may occur over time, including an initial disclosure
and a post analysis disclosure. Identiable
patient or sta information should only be used
or disclosed with patient or sta consent, or if
there is some other legislative means (for patient
information refer to Part 7, Division 2,s.145, 146 or
153 of the Hospital and Health Boards Act 2011).
Disclosure to the community should not occur
until appropriate expert advice (including legal) is
sought to ensure there is no inadvertent release of
private/condential information.
There are a variety of guidelines to assist in
clinician and formal open disclosure processes
(roles, responsibilities, what to disclose and how):
• Patient Safety and Quality, Clinical Excellence
Queensland
• Open Disclosure Guide
• Australian Commission on Safety and Quality in
Healthcare
This structured open disclosure process supports
the transparent discussion between the patient
and the patient’s family or carer, senior clinician
and representatives of the health service about
the clinical incident, that resulted in harm which
was not reasonably expected as an outcome of the
health care provided. The overarching aim of open
disclosure is to ensure patients and their families
or carer have a reliable, caring and eective means
to receive honest and factual information about the
clinical incident associated with their healthcare.
(11)
Importantly, empathetic and timely disclosure can
help patients, families, carers and sta deal with
the consequences of a clinical incident.

Step 2 – Immediate response | 33
Handy tip
Open disclosure is comprised of two
components: clinician disclosure (CD) and
formal open disclosure (FOD).
Clinician disclosure is dened as an
informal process where the treating clinician
informs the patient/ family/carer of what
has occurred, and apologises for the harm
caused or adverse outcome. In general, it
is used for the initial disclosure aer the
incident and may be all that is required for
less serious events.
(12)
Formal open disclosure (FOD) is the
structured process to ensure communication
between the patient/ family/carer, senior
clinician and the organisation in response
to the most serious clinical incidents. To
enable this process, an open disclosure
team involving members of the treating team
and the organisation executive is activated
prior to the meeting with the patient/family/
carer. A senior clinician who is not part of
the treating team and trained as an open
disclosure consultant (ODC) leads this team
through the FOD process. FOD involves
multidisciplinary discussions that support
clinical incident management processes and
provides a format that facilitates and enables
open communication between patients,
families, carers, clinicians, senior clinical
leaders and hospital executives.
(12)
http://
qheps.health.qld.gov.au/psu/od
Health Service organisation are required to
implement open disclosure as part of the
Clinical Governance standard in the National
Safety and Quality Health Service Standards
(second edition), developed by the
Australian Commission for Quality and Safety
in Health Care. Open disclosure is described
in Standard 1.12.
Oen practical support is needed, and
contacts should be provided to the patient,
family, carer and clinicians so that those
who may have suered emotionally and
physically can receive early assistance.
Disclosure, expressions of compassion and
oering an apology are important elements of
communication, helping both patient, families,
carers and clinicians in healing and in restoring
trusting relationships.
(12,13)
Reduce risk of imminent
recurrence
Local actions to reduce the risk of imminent
recurrence may need to be taken immediately;
additional actions typically follow aer a
more thorough analysis has been undertaken.
Patients, families, carers and sta should be
informed of immediate actions.
Handy tip
A senior clinician should attend the scene
of the incident to support the immediate
response as soon as possible aer the
incident. They need to assess the patient
and/or family, the sta involved, and any
immediate issue, concerns or risks. They
need to record the preliminary facts.

34 | Best practice guide to clinical incident management Second edition - January 2023
Step 3: Prepare for analysis
Preliminary assessment
In order to determine appropriate follow-up to an
incident, including the need for analysis, an initial
assessment or preliminary fact-nding process
is needed. The key outcome of this step will be a
high-level sequence of events and documentation
of known facts related to the incident. There
will be organisational variation as to how the
individuals responsible for the initial fact nding
conduct this process and how the information is
incorporated into the organisational response to
an incident. It is recommended that individuals
responsible for the preliminary assessment of
clinical incidents be provided with education
in incident analysis, including an introduction
to systems thinking, human factors principles
and other essential concepts. Sta undertaking
reviews should also have access to organisational
mechanisms/tools to assist with the identication
of key trends in incident prevalence at the local
level including any contributing factors.
Once the preliminary (or triage review)
assessment phase is complete, a determination of
next steps follows. In some cases, it will be clear
that further system-based analysis is needed,
while in others an accountability review or
alternative quality improvement process may be
more appropriate.
The Just Culture Approach has been adapted for
Queensland Health and replaces the incident
decision tree that was based on the work of
Professor James Reason and the previous
National Patient Safety Agency. (Appendix
C) This Just Culture Approach should not be
used routinely; only when there is evidence
or a suspicion that an individual may require
support or as part of an individual practitioner
performance accountability investigation to
enable constructive conversations.
(45)
If based on the preliminary understanding
of what happened (from incident report and
initial analysis of facts) it is determined that an
analysis is required, then it is usually at this
point that a system-based method of analysis
is determined. Three types of incident analysis
are described in this guide—comprehensive,
concise and multi-incident. Determination of
the appropriate method is made using a range
of criteria. This decision is usually made jointly
by the manager involved, together with the
quality and safety leads, the clinical leads,
senior leaders and others as dened in the
organisational policies and procedures. Each
incident analysis method includes a systematic
process to identify what, how and why the
incident happened, what can be done to reduce
the likelihood of recurrence and make care safer
and share learnings.
– Preliminary assessment
– Select an analysis method
– Identify the team
– Coordinate meetings
– Plan for/conduct interviews

Step 3 – Prepare for analysis | 35
Methods of incident analysis -
overview
In numerous consultations with patient safety
experts and those engaged in incident analysis,
it became clear that one method of incident
analysis is not necessarily appropriate for
all types of incidents. A literature analysis
and environmental scan of analysis methods
conrmed the emergence of a variety of methods
for clinical incidents analysis in healthcare. A
range of methods is important for users, who
can select the one most appropriate for their
healthcare facility, context, skills, resources
and type of incident. The methods included in
this Guide have been designed to be flexible to
accommodate use in dierent care settings.
This Guide oers three methods in total: there
are two methods for analysing individual
incidents (comprehensive and concise) and one
method for multiple incidents (multi-incidents).
All three methods aim to determine what
happened, how and why it happened, what can
be done to reduce the risk of recurrence and
make care safer, and what was learned.
Regardless of the method used, the basic
principles and steps in the analysis process
are the same; however, the level of detail and
the scope of the analysis will dier with each
method. Below is a short description of each
method, followed by guidance on how to select
the appropriate method to analyse a particular
incident or grouping of incidents.
Comprehensive analysis is usually used for
complicated and complex incidents that
resulted in catastrophic/major harm, or the
signicant risk thereof. Multiple sources of
information are consulted, including interviews
with those directly or indirectly involved in
the incident as well as experts, supplemented
by a literature analysis. A signicant amount
of time and resources (human and nancial)
can be invested to conduct the analysis. The
nal report produced will include a detailed
sequence of events of the facts, contributing
factors and their influences, ndings from the
literature search/ environmental scan, context
analysis, recommended actions, and where
applicable, implementation, evaluation and
dissemination plans. Members of the senior
leadership of the organisation need to be
kept apprised of progress and may be directly
involved in the process.
Concise analysis is a succinct, yet systematic
way to analyse incidents with no, low or
moderate severity of harm. Generally, the
incident and analysis process is localised to
the unit/program where care was delivered.
The sources of information consulted are the
available reports, supplemented with a small
number of select interviews and a targeted
analysis of other sources of information. The
analysis is completed in a short interval of time
by one or two individuals. At the end of the
analysis, a report is produced that contains the
facts (including a brief sequence of events),
contributing factors, a brief context analysis and
where applicable, recommended actions and
plan for evaluation and dissemination.
Multi-incident analysis is a method for
analysing several incidents at once instead of
one by one, by grouping them in themes (in
terms of composition or origin). Multi-incident
analysis can be used for incidents that resulted
in no, low or medium severity of harm as well
as near misses that took place at any location
in the organisation (possibly in a short interval
of time). It can also be used to analyse a group
of comprehensive and/or concise analyses.
This method of analysis can generate valuable
organisational and/or sector-wide learning that
cannot be obtained through the other methods.
Detailed information on each of these incident
analysis methods is set out in Step 4.
Selecting a method of incident
analysis
The Severity Assessment Code (SAC) rating is
the way patient safety events are classied in
the Queensland Health public system. Refer to
Appendix F (Severity assessment code (SAC)
matrix).
The Patient Safety Health Service Directive
requires that all SAC1 clinical incidents
and analysis reports are submitted to the
Patient Safety and Quality, Clinical Excellence
Queensland within 90 days of being reported in
RiskMan. Each analysis report must contain:
• a factual description of the event
• the factors identied as having contributed
to the event
• recommendations to prevent or reduce the
likelihood of a similar event happening
again.

36 | Best practice guide to clinical incident management Second edition - January 2023
The method of analysis is a matter for local
policy and need not be determined by the SAC
rating alone. There are situations where an
incident with a high level of harm SAC rating
may be more appropriately analysed with a
concise analysis and other situations where
an incident with a low level of harm SAC rating
requires a comprehensive analysis.
When selecting a method to analyse incidents,
consider a number of criteria including:
• severity of the incident
• probability of recurrence
• complexity of the factors that appear to have
influenced the incident on the organisation
(unit, organisation or system)
• other contextual factors (preliminary
assessment, frequency of occurrence,
regulatory mandates, internal or external
pressures).
Table 3. Criteria to consider in selecting an incident analysis method
Criteria Comprehensive
analysis
Concise analysis Multi-incident
analysis
Severity assessment code SAC1 and some SAC2
SAC2 and some SAC3,
SAC4
SAC1, SAC2, SAC3 and
SAC4
Severity and probability
(see Table 4)
A and some B B and some C A, B and C
Complexity level (degree
of agreement, certainly,
number of interactions)
Complicated, complex Simple, complicated
Simple, complicated or
complex
Area of impact
Team, unit / program,
organisation, system
Team, unit /
program, or possible
organisation
Team, unit / program, or
possible organisation,
system, sector, industry
Context – internal and
external pressures
High Low Low, medium or high
Resources required /
available (time, nancial,
human)
Moderate to extensive Limited Moderate to extensive
In the case of near misses or incidents where
the outcome is not known at the time of
the analysis, the worst possible outcome
should be considered. Additionally, factors
such as incident analysis skills and limited
resources available to analysis teams require
consideration. These criteria are summarised in,
Tables 3 & 4, and Appendices C (A just culture
approach), F (Severity assessment code (SAC)
matrix, G (Guide to level/type of analysis). See
Step 1 for descriptions of complexity, area of
impact and context.

Step 3 – Prepare for analysis | 37
Severity/probability matrix score
Table 4 below, is another type of stratication
tool that links the severity of the clinical
incident with its probability of recurrence. The
tool applies to all incidents (harmful, no harm
and near misses).
Table 4. Severity versus probability matrix
(46)
Probability
Catastrophic
Major
Moderate
Minor
Frequent A A B C
Occasional A B C C
Uncommon A B C C
Remote A B C C
Key factors for the severity categories are extent
of injury, length of stay, level of care required
for remedy and actual or estimated physical
plant costs. For harmful and no harm incidents,
assign severity based on the patient’s actual
condition. If the event is a near miss, assign
severity based on a reasonable ‘worst case’
system level scenario. For example, if you
entered a patient’s room before they were able
to complete a lethal suicide attempt, the event
is catastrophic, because the reasonable ‘worst
case’ is death of the patient.
In order to assign a probability rating, it is ideal
to know how oen it occurs at your facility.
Sometimes the data will be easily available
because they are routinely tracked (e.g.
falls with injury, adverse drug events, etc.).
Sometimes, getting a feel for the probability of
events that are not routinely tracked will mean
asking for a quick or informal opinion from sta
most familiar with those events. At times it will
have to be your best educated guess.
(46)
It is important to note the analysis methods
presented here are not mutually exclusive.
For example, contributing factors derived
during a concise incident analysis could
also be the foundation for a comprehensive
or multi-incident analysis. In the event that
a comprehensive analysis was recently
conducted, and a new similar incident occurs,
a concise incident analysis may be sucient to
determine if any new contributing factors need
to be addressed.
Level/type of analysis based on
the degree of harm
There a number of triggers for review and
an analysis should be conducted where
appropriate to identify learning points from
patient safety incidents, claims, complaints
and concerns. In determining what type/level
of analysis is appropriate, it is important to
consider the degree of harm.
Hospital and Health Services may have their
own policies on the type of review each
SAC rating requires, and may stipulate the
associated reporting requirements.
There may be situations where a clinical
incident with a low level of harm SAC rating
requires a comprehensive analysis based on the
level of risk. When considering if an analysis is
required there a number of criteria to consider
including:
• severity of incident
• probability of recurrence
• complexity of the factors that influenced the
incident
• other contextual factors (preliminary
assessment, frequency of occurrence,
regulatory mandates, internal or external
pressures).
Appendix G (Guide to level/type of analysis)
provides suggestions on what might be
considered the appropriate the level/type of
analysis required for a clinical incident based
upon the degree of harm and assessed risk.

38 | Best practice guide to clinical incident management Second edition - January 2023
Identify the team and the team
approach
Typically, an analysis team facilitator (with
expertise in analysis such as the patient safety
ocer or equivalent) and an executive leader
(with operational responsibility such as the
Service Executive Director who understands
and supports the analysis) share primary
responsibility for conducting, coordinating
and reporting on each analysis in accordance
with applicable organisational policies.
Decisions about the involvement and timing
of involvement of various individuals are likely
to vary from organisation to organisation and
will be influenced by the incident context, as
well as local culture and previous experience.
In considering the involvement of various
individuals, it is important to clearly dene the
roles and responsibilities of everyone who will
participate in the analysis process.
Not all team members are required to be
involved in all aspects of the analysis. For
example, clinicians directly involved and
patients/family/carers may participate in the
information gathering stage and provide further
input into solution development. Other direct
care sta may participate in the actual analysis
stage. Senior leadership representatives may
actively participate in the analysis or support
the process at arms length. Support and
involvement of senior operational leaders in
the analysis process helps to demonstrate a
commitment to change at the highest levels of
the organisation and also helps to ensure that
recommended actions are developed within
the context of the broader organisation. It is
also useful to involve relevant external experts/
consultants with specialised knowledge of
the system undergoing analysis and/or the
analytical process (especially for comprehensive
analyses). For additional detail on team roles
and management, see Appendix A (Analysis
team membership, roles and responsibilities).
Handy tip
The analysis team is the group charged
with incident analysis. Refer to Appendix
H (Sample analysis team charter). Other
individuals may be involved in the analysis
process (e.g. through interviews, meetings,
fact nding and/or consultations).
The team composition will vary depending
on the incident and applicable legislative
protection as well as on the organisation’s
approach to analysis (e.g. one individual
may conduct interviews and fact nding then
bring the group together to conrm and gain
consensus on facts, contributing factors,
recommended actions, or the entire process
may be a team eort).
The success of the analysis depends on the
involvement of those who provided care as well
as the patient or family. There may be a number
of sta and/or agencies who will have the
responsibility for reviewing clinical incidents
that occurred during a during a patient’s journey
across multiple health organisations and/
or services. The process of engaging the key
stakeholders is referred to in this Guide as a
multi-agency review.
A multi-agency review may be indicated
when a clinical incident occurs during a
patient’s journey across a number of health
organisations, health providers and/or
retrieval services. The complexity associated
with dierent transitions in care may have
contributed to an adverse outcome for the
patient. An investigation into a clinical incident
which involves more than one site, service
or stakeholder is considered a multi-agency
review.
Considerations relating to the patient/family/
carer may also include ensuring they have the
time needed to emotionally process what has
happened or an immediate need to make care
or funeral arrangements, the patient and/or
family/carer may not be ready and/or able to be
involved in the analysis.

Step 3 – Prepare for analysis | 39
Being respectful of the needs of the
patient/ family/carer and keeping the lines
of communication open may enable their
participation at a later time. The same can
be said of healthcare workers who were
directly involved in the incident. However, it
is essential that the patient/family/carers,
involved healthcare workers and their relevant
healthcare agency be part of the initial process
of information gathering.
The key benets are:
• An open and sincere partnership with all
involved in the incident can result in healing
relationships, regaining trust in each other
and the system, and improving the wellbeing
of all participants.
• When the team comes together, they may
discover new information not previously
known by all members of the care team.
• Analysis is an invaluable method that
permits those involved in an incident an
opportunity to help reveal information
that may lead to solutions to make care
safer. This allows all involved to impact the
system they work in and to take ownership
of changes, rather than feeling that changes
are forced upon them.
Handy tip
There are several types of analysis teams:
• External—all team members are from
outside the organisation.
• Internal—all members of the team are
employed by the organisation.
• Internal with external support—most
are internal sta, and few are external
members..
The context and circumstances surrounding
each incident are dierent and careful
consideration should be given to all
relevant factors before deciding on how to
approach the analysis. It is important that
organisations proactively develop a plan on
how to approach the analysis that will help
teams respond quickly and eectively when
an incident occurs.
Analysis involving internal teams working
collaboratively with internal and/or external
experts (multi-agency review) are benecial
to the culture of the organisation as well as
in rebuilding trust.
Coordinate meetings
It is common for an analysis facilitator to
collaborate with the analysis executive team
leader to conduct background work and collect
the necessary information for the analysis
(e.g. health record, sequence of events,
relevant policies and procedures, evidence-
based guidelines, etc.). The full analysis team
is convened at a mutually agreeable date and
time. It is recommended that all documentation
provided to the team during meetings, include
the sequence of events should be tracked and
returned to the facilitator at the end of the
analysis.
An experienced facilitator will be able to
anticipate and manage issues that arise during
the analysis process. Keys to success include
providing a comfortable, private setting (ideally
away from the care area where the incident
occurred), setting ground rules for discussions
and ensuring necessary information is readily
accessible.
Some suggested ground rules include the
following:
• respect for individuals
• respect for opinions expressed
• equal participation by all
• respect for condentiality of the discussions
• ask questions to clarify rather than
challenging others
• decisions by consensus.
A checklist can help the analysis facilitators to
prepare for and manage meetings eectively.
Refer to Appendix I (Team management
checklist) for further information.
Plan for and conduct interviews
Interviews are key to collecting information
for analysis and also help to support those
directly involved in the incident. An interview
is oen the rst opportunity that a patient/
family/carer or healthcare clinician has to share
their detailed perspective about the incident.
The interview process may cause anxiety and
further distress—therefore it is important to be
respectful and supportive of those involved and
be clear about the purpose of the interview and
what will be done with the information provided.

40 | Best practice guide to clinical incident management Second edition - January 2023
Interviews should be conducted as soon as
reasonably possible aer the incident for
two reasons. Firstly, memories fade quickly
and important details may be lost over time.
Secondly, as individuals involved in the incident
discuss their recollections with one another,
versions may blur together and the opportunity
to obtain unique perspectives and details may
be missed.
It is recommended that individual interviews
occur with all sta involved in the incident as
well as individual or group interviews with the
patient and family members as appropriate.
A cooperative approach is encouraged, using
open-ended questions. Individuals should be
asked to ‘tell their story’ and possibly re-enact
the incident or portions of the incident. If
possible, do not interrupt while the interviewee
is telling their story as this increases the
likelihood that parts of the story may be
missed. Instead hold the questions and further
clarication until the story has been told. It is
helpful to ask individuals being interviewed if
there are any factors they think contributed to
the incident (e.g. environmental factors such as
lighting, noise levels, time of the day, workload
etc.) as well as factors they feel mitigated the
outcome of the incident (e.g. what went well).
It is important to record the interview in
a comfortable way, noting that video or
audio recording can increase anxiety for
the interviewee and are not generally
recommended. You will also need to ensure that
you seek the interviewee’s permission to record
the interview.
It is preferred that interviews be conducted
one person at a time so that individual
perspectives about the incident are well
understood for their nuances and unique
points of view. Interviewers should provide
information about the analysis process and
encourage further follow-up if the interviewee
recalls any other details, they feel are
important to understanding the incident aer
the interview has been completed.
Finally, sincerely thank people for helping to
provide an understanding of the incident and
ensure their questions about the process are
answered before drawing the interview to a
close.
Some further guidelines that can be used
or adapted by organisations are included in
Appendix J Investigative interview guidance
(cognitive type interview).
Handy tip
A common problem with any type of
analysis is the failure to establish all the
facts. Good witness interviews will help
answer the questions:
• What should happen?
• What usually happens?
• What happened in this case?
• What is the variance?
Make sure
Before commencing interviews, the
investigator must explain in precise detail
the limits on condentiality, what access
mechanisms apply and what protections
(if any) apply to sta who give a statement
and whether they have the option to decline
to participate if they consider they will be
exposed to action.
Avoiding cognitive traps
Cognitive biases are implicit mechanisms that
influence reasoning and decision making
(47)
and
as a result, impact the analysis process. Bias
can influence the team in a number of ways,
resulting in the following:
(48)
• oversimplication of what contributed to the
outcome
• overestimation of the likelihood of the
outcome
• overrating the signicance of some factors
and actions
• misjudging the prominence or relevance of
facts/data
• premature completion of the analysis
process
• overcondence in interpretation of known
information.

Step 3 – Prepare for analysis | 41
Awareness of bias needs to be cultivated in
those leading and participating in the analysis
and every eort should be made to recognise
and reduce the influence of bias. One approach
to reducing bias is to include people and/
or consumer representatives on the analysis
team who are not aware of the details of the
incident under analysis, or who are naïve
to the processes involved. Another is for all
participants to be encouraged to listen actively
to the contributions of each team member
and avoid jumping to conclusions. Additional
techniques include the use of guiding questions
(Appendix J) and the constellation diagram
(Appendix E) as decision aids— these tools will
help the team to explore multiple categories
of contributing factors and understand their
interconnections. Using a combination of
dierent approaches is encouraged.
Rarely are all of the important contributing
factors immediately known and as a result,
oen the initial perceptions are found to be
incorrect once a more thorough analysis that
considers the whole system (work environment,
organisation, context) has been undertaken.
(49)
Identifying and addressing potential biases in
the analysis supports a just and safe culture and
a learning environment.

42 | Best practice guide to clinical incident management Second edition - January 2023
This Guide oers three methods total: two
methods for analysing individual incidents
(comprehensive and concise) are included
along with another method suitable for review
of multiple incidents (multi-incidents). All
methods aim to determine what happened,
how and why it happened, what can be done
to reduce the risk of recurrence and make care
safer and what was learned.
Regardless of the method used, the basic
principles and steps in the analysis process
are the same; however, the level of detail and
the scope of the analysis will dier with each
method. This section sets out each analysis
method in detail. In summary, the following
methods to be discussed are:
• comprehensive analysis
• concise analysis
• multi-incident analysis.
For each of the analysis methods, there are a
range of dierent tools/templates available; a
comprehensive analysis can be conducted with
a RCA, London Protocol and the Human Error
and Patient Safety (HEAPS). Of these, there are
more than 40 RCA techniques described in the
literature. The London Protocol and HEAPS have
been modied by dierent agencies to suit
their requirements. Individual facilities have
designed their own concise analysis templates.
– Understand what happened
– Determine how and why it
happened
– Develop and manage
recommended actions
Step 4: Analysis process

Step 4 – Analysis process | 43
Comprehensive analysis
A comprehensive, or detailed analysis of a single incident is generally undertaken when permanent
harm or death has occurred (or a signicant risk thereof), the incident is complicated or complex,
the area impacted is at micro, meso, or macro level, and/or the contextual pressures are high. See
Appendix K (Case study-comprehensive analysis: resident absconds from a residential aged care
facility) and Figure 5 below for the flow diagram.
Figure 5. Flow diagram for comprehensive analysis
Follow through—implement, monitor, assess.
Close the loop—share what was learned (internally and externally).
Develop and manage recommended actions
What can be done to reduce the risk of recurrence and make care safer?
• Develop recommended actions
• Suggest an order of priority
• Prepare and hand-o report for endorsement by leadership as appropriate
• Allocate timeframes and individuals responsible for each recommended action
• Include an evaluation strategy for each recommended action.
Determine how and why it happened
• Analyse information to identify contributing factors and the relationship/s among them:
− use systems theory and human factors
− use diagramming:
• describe the incident and outcome
• identify potential contributing factors
• dene relationships between and among potential contributing factors
• identify the ndings (can be highly relational)
• conrm the ndings with the team.
• Summarise ndings.
Before the incident Immediate response Prepare for analysis
• Preliminary assessment
• Select analysis method
• Convene an interdisciplinary team
• Coordinate meetings
• Plan for and conduct interviews
Understand what happened
• Gather information:
− analyse incident report
− analyse additional information:
• health record
• interviews with all individuals directly/indirectly involved (including patient/family/carer)
• Visit the location where the incident occurred; if possible simulate the incident
• Examine any items involved in the incident
• Create a detailed sequence of events
• Analyse supporting information—policies, procedures, literature, environment scan,
previously reported incidents, consultations with colleagues or experts.
Analysis process
COMPREHENSIVE

44 | Best practice guide to clinical incident management Second edition - January 2023
Steps in conducting a
comprehensive analysis
What happened?
Gathering information
The team’s rst priority is to gather information
relevant to the incident. This stage of the
process is intended to answer the ‘what
happened?’ question and will begin to elucidate
how the incident occurred. The importance
of a thorough information gathering phase
cannot be over-emphasised. The team cannot
proceed to understand the contributing factors
related to the incident if they do not have a
clear understanding of the circumstances
surrounding the incident. A systematic process
for assessing information needs and gathering
information will help to ensure that the analysis
is both thorough and credible. It may be
helpful for organisations to develop a template
or checklist to help the facilitator prepare
information for analysis by the team.
Handy tip
An analysis team should:
• analyse and interpret all sources of
documented evidence
• if able, interview patient/family/carer
• identify and interview all relevant sta
• visit the physical area where the incident
occurred
• inspect equipment/tools
• take photos
• analyse evidence of policies,
procedures, standards and relevant
literature
• have relevant evidence of trigger
questions within the analysis
• if indicated, must identify and interview
experts and seek external opinion/s.
Analyse incident report
The incident report is typically the rst formal
summary of information related to an incident
and is based on an initial understanding of
the facts. Analysis of information provided in
the incident report will direct the preliminary
analysis approach. Other sources of
information that may trigger the initiation of
a comprehensive analysis include patient
concerns, information identied with the use of
trigger tools, audits, attention from the media/
general public or coroner’s reports.
Analyse additional information
In addition to gathering and analysing the
health record in detail, it is important to
interview all clinicians and others who were
directly or indirectly involved in the incident,
including the patient/ family/carer. Where
possible, it is recommended that the team visit
the location where the incident occurred. When
a physical visit is not possible, photographs
and videos are recommended. During the
visit, important details or other contributing
factors that people did not remember or did not
recognise as important can be identied. Items
that may have been involved in the incident
(e.g. syringes, labels, devices, medications)
need to be secured at the time the incident is
identied. If the original items are not available
the team should be given access to similar
appropriate items to assist them to understand
what, how and why it happened.
Create a detailed sequence of events
When all the information is gathered and
analysed, the team should be able to ll in
identied gaps in the initial understanding of
the incident provided by the incident report or
other triggering mechanism, and then create
a detailed sequence of events. It is common
to provide this information in the form of a
narrative sequence of events description.
(Refer to Appendix K case study). The detailed
understanding will enable collation of the
information from various sources, including
the health record and interviews with key
individuals. As the care of the patient aer the
incident may be relevant to mitigation of harm
from the incident, it is appropriate to include
details related to patient management once the
incident was discovered.

Step 4 – Analysis process | 45
Because the team will use the detailed
sequence of events as a starting point for
identifying system- based factors underlying the
incident, it is crucial that the sequence of events
include only the actual facts or processes as
they occurred, and not what was supposed
to happen. The detailed understanding of
the incident is nearly always dierent from
the initial information available, reinforcing
the importance of fully investigating the
circumstances of an incident designated for
comprehensive analysis.
Handy tip
The detailed sequence of events should
show the comprehensive sequence
of events—presenting facts without
speculation. Include key objective data
including signs and symptoms, clinical
assessments, analysis results, treatment
provided and evaluation of progress.
Do not include entries which have
information that is not relevant to the
incident.
Analyse supporting information
An incident analysis should prompt the team to
analyse existing policies and procedures. This is
important for two reasons—rstly, it establishes
the documented organisational expectations
related to care; and secondly, it provides a
baseline to evaluate current organisational
practices in relation to current evidence and
leading practice guidelines.
An environmental scan of current practices in
similar organisations and a literature analysis
(scope will vary depending on the incident)
will help to provide context for the incident
as well as determine if there are any leading
practices or evidence-based guidelines
relevant to the incident.
Previous similar incidents or near
misses (reported internally or by other
organisations) may also be identied.
Incident descriptions and information about
actions taken and challenges encountered
by other organisations that have dealt
with similar issues can assist the team in
understanding contributing factors and
developing recommended actions. It is
suggested that as part of this process that
you also review statewide Patient Safety
Alerts and Advisories as well as national and
international generated alerts to inform your
information base.
Sometimes, unique incidents have no literature
citations available. In these cases, consultation
with the Patient Safety and Quality, Clinical
Excellence Queensland may help determine
if the issue in question has been previously
observed in practice, but not published.
How and why it
happened
As the team begins to understand the
circumstances of the incident, contributing
factors and relationships will begin to emerge.
A series of investigative categories and guiding
questions (refer to Appendix L Incident analysis
guiding questions) have been adapted from
work by international experts in incident
analysis
(49,50,51,52)
to provide a starting point for
analysis and assist teams to ensure all relevant
aspects of the incident have been analysed in
detail during the interviews and the analysis
phase. This portion of the analysis is about
answering the ‘how and why it happened?’
question.
The focus at this point is to recognise any
related system issues that may have contributed
to the incident. While it is human nature to
identify factors at the intersection between the
patient and clinician (e.g. the micro level), the
goal of the analysis is to move the team towards
the meso, macro and mega levels of the system
(e.g. processes, policies, environment) to
ensure all the contributing factors are identied.
During this phase of the analysis, the team will
need to ask questions such as, ‘what was this
influenced by?’ and ‘what else aected the
circumstances?’ The team will use the detailed
sequence of events of the incident, supported
by the principles of systems and human factors
theory, to answer these questions in order to
identify the contributing factors.

46 | Best practice guide to clinical incident management Second edition - January 2023
Handy tip
The analysis team must dene the real
problem to be eliminated to prevent a
similar incident occurring again. The
problem statement is best constructed
as a short verb, noun statement. Eective
problem resolution begins with agreeing
upon a denition of the problem. For
example, Problem statement: Patient
fractured his hip.
Use systems theory and human
factors
There has been a major change in patient
safety culture and thinking about incident
causation in the health system in the last 20
years with the application of systems theory
and human factors. Applying systems theory
and the principles of human factors can assist
in answering the above questions by focusing
the analysis on the system-based contributing
factors. In particular, human factors provide the
tools, methods and theories to approach these
questions. The goal when applying human
factors is to focus not just on the human or
the system alone, but rather the interaction
between the human and the system, and to look
for the factors that influence that interaction.
These influencing factors may be related to the
equipment, the task and the work environment,
in addition to inherent human characteristics
and limitations.
Various human factors methods can be
employed at this stage of the analysis process
to help answer the question, ‘how and why it
happened?’. They range in complexity, time and
resources needed and expertise/ experience
in human factors required. Three methods are
described in Appendix M (Three human factors
methods that can be used in incident analysis);
cognitive walkthrough, heuristic evaluation
and usability testing. All three methods assist
in examining the human system interaction in
detail.
• With cognitive walkthrough, the easiest and
most cost eective method to employ is to
ask participants to ‘think out loud’ while
simulating the tasks that were involved in
the incident.
• In a heuristic evaluation, an audit is carried
out on the various parts of the system (such
as equipment, paper forms, and computer
systems) that were used in the tasks that
were part of the incident. The audit is
used to determine if human factors design
principles were violated and as such, may be
identied as possible contributing factors in
the incident. Heuristic evaluation requires
an understanding of human factor principles
as they apply to dierent systems (e.g.
computer systems).
• Usability testing provides an observation
of the human system interaction with
equipment, paperwork, or process (similar to
a simulation). Participants are asked to carry
out a set of tasks in a simulated environment
and given the scenario as it occurred during
the incident. Some level of human factors
training is needed in order to plan and
execute usability tests, and to interpret the
results. If conducted correctly, the usability
test provides important information about
how the human system interaction occurs in
the real-world setting.

Step 4 – Analysis process | 47
Using diagramming
One tool that can assist the team to work through the questioning process is the use of diagramming.
Diagrams can help teams to identify and understand the interrelationships between and among
contributing factors. Diagramming shis the focus away from individual performance, towards system
performance and underlying factors, helping to clarify team understanding and ensuring a thorough
analysis of the incident.
Ishikawa, also called Fishbone, (Figure 6) and Tree diagrams (Figure 7) are utilised to support
analysis, however both these types of diagrams have limitations.
(53,54)
Ishikawa diagrams are helpful
for brainstorming and clustering factors, but do not easily illustrate complex relationships between
factors. Tree diagrams have been perceived as too linear and their top-down approach can be
misleading in terms of relative importance of identied contributing factors.
Figure 6. Ishikawa diagram
Communication Training Fatigue/Scheduling
Policies/procedures
Environment/
equipment
Barriers
Critical incident
Figure 7: Tree diagram
Action or
condition
Action or
condition
Root cause
caused by
caused by
caused by
caused by
caused by
caused by
caused by
Harmful
outcome
Action or
condition
Action or
condition
Incident
Action or
condition
Action or
condition
Action or
condition
Action or
condition
Root cause
Root cause
caused by caused by
caused by caused by
caused by caused by
To attempt to address the advantages and limitations of these two types of diagrams, the features of
each were blended into an innovative diagram that evolved from the shbone and tree diagrams into
what is called a constellation diagram, illustrated in Figure 8 on the following page.

48 | Best practice guide to clinical incident management Second edition - January 2023
Figure 8. Example of a constellation diagram
Incident:
Outcome:
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Care team
Work
environment
Organisation
Equipment
Patient
Other
Task
Factor
Factor
Factor
Factor
Factor
Finding
Finding
Finding
Finding
Finding
Finding Finding
Finding
Finding
Finding
Factor
Factor
Factor
Factor
Finding
Factor
Finding
Additional details and instructions for developing a constellation diagram are provided in Appendix E
(Creating a constellation diagram).
Regardless of the type of diagram used to support incident analysis, the basic steps will be similar:
• describe the incident
• identify potential contributing factors
• dene interrelationships between and among potential contributing factors
• identify the ndings and conrm the ndings with the team.

Step 4 – Analysis process | 49
Summarise ndings
Once the team has completed the analysis,
a summary of what was found is prepared to
clearly articulate the contributing factors related
to the incident. The contributing factors are
provided in the analysis report as a series of
‘statements of ndings’.
The statements of ndings describe the
relationships between the contributing
factors and the incident and/or outcome. The
statements focus on the contributing factors
and should be as specic as possible (note that
there could be a group of factors that together
contributed to the incident or outcome).
For those familiar with RCAs, the statements of
ndings have been adapted from the previous
‘causal statements’.
Formulation of the statements may be assigned
to a sub-group of the analysis team and
analysed with the full team at a subsequent
meeting.
The statements of ndings describe the
relationships between the contributing
factors and the incident and/or outcome. The
statements focus on the contributing factors
and should be as specic as possible (note that
there could be a group of factors that together
contributed to the incident or outcome).
The suggested statement format is:
‘The contributing factor/s, within the context
of the incident, increased/decreased the
likelihood that this outcome would occur’.
Refer to Appendix N (Developing a statement
of ndings), for a template adapted from ACHS
Improvement Academy. Two sample statements
of ndings are also provided with dierent
scenerios used within the template.
A well constructed constellation diagram
will assist in the development of statements
of ndings, working from the outside of the
diagram, back towards the centre. Examples
of statements of ndings can be found in the
case examples in Appendix K (Case study—
comprehensive analysis: resident absconds
from a residential aged care facility) and
Appendix O (Case study-concise analysis:
medication incident).
The Centre for Healthcare Engineering and
Patient Safety, University of Michigan references
the 5 Rules of Causation.
Handy tip
Hints for well constructed statements of
ndings using Five Rules of Causation
1. A statement of nding should clearly
show the link between the contributing
factor and the harm aected by the
patient.
2. A statement of nding should use
specic and accurate descriptors for
what occurred, rather than negative and
vague words.
3. A statement of nding should identify
the preceding system contributing
factors, not the human error.
4. A statement of nding should identify
the preceding system causes of
procedure violations.
5. Failure to act is only causal when there is
a pre-existing duty to act.
What can be done
to reduce the risk of
recurrence and make
care safer?
The ultimate goal of incident analysis is to take
action to reduce the risk of recurrence and make
care safer. Step-by-step guidance on developing
and managing recommended actions is
included under ‘Recommended actions’.
What was learned
Additional attention is needed to identify
learning from incidents within and outside
individual practice settings and to share
learning so others can take appropriate steps
to provide safeguards in their own settings.
Step 6 provides guidance on continuous
organisational learning and sharing results.

50 | Best practice guide to clinical incident management Second edition - January 2023
Concise analysis
Given the complexity of the healthcare environment and the signicant resources required for
comprehensive incident analysis, healthcare
leaders and patient safety experts have
included a more concise method of incident
analysis to help meet the need for timely
and accurate action on a larger number of
incidents. It is recognised that there are various
types of analyses that may be appropriate in
this respect; morbidity and mortality (M&M)
reports or the use of specially designed report
templates for recurring incidents.
Adopting a concise incident analysis, whilst
conducted in a dierent format remains
consistent with the principles and methodology
of incident analysis, including the employ of a
systems approach and consideration of human
factors. (Appendix M details three human
factors methods that can be used in in incident
analysis).
Utilising a concise analysis requires a conscious
and deliberate decision to focus primarily on
four aspects:
1. the agreed upon facts
2. key contributing factors and ndings
3. actions for improvement (if any) and
4. evaluation.
Refer to Figure 9 (Flow Diagram for Concise
analysis)
Refer also to Table 5 for comparing the
characteristics of concise and comprehensive
incident analysis
55
and see Appendix O for a
case study using the concise method.
If at any point during the concise
analysis the facilitator feels that
the analysis should be escalated to
comprehensive, they should discuss
this with their line manager/delegate
and seek further instructions. Note: All
types of analysis benet from a team
approach.

Step 4 – Analysis process | 51
Figure 9. Flow diagram for concise analysis
Follow through—implement, monitor, assess.
Close the loop—share what was learned (internally and externally).
Develop and manage recommended actions
What can be done to reduce the risk of recurrence and make care safer?
• If there is sucient evidence to formulate recommended actions:
− include known or easily identiable actions for improvement (evidence based where possible)
− briefly describe an evaluation strategy
− provide applicable decision maker for decision and action.
Before the incident Immediate response Prepare for analysis
• Preliminary assessment
• Select appropriate analysis method.
• Identify analyser (typically one person)
Understand what happened
• Develop initial understanding of event:
− gather facts: sucient information to understand what happened
− informal discussion with patient/family/carer, clinician/s, manager/s and/or experts
in the process or equipment related to the incident.
Analysis process
CONCISE
Determine how and why it happened
• Analyse information to identify contributing factors and the relationship/s among them:
− se systems theory and human factors
− describe the incident and outcome
− use the guiding questions to briefly explore all the categories
− dene relationships between potential contributing factors
− identify ndings (can be highly relational)
• Summarise ndings
• Determine if there is sucient evidence to formulate recommended actions.

52 | Best practice guide to clinical incident management Second edition - January 2023
When to use a Concise approach
A concise approach is most commonly used for incidents or concerns that resulted in minimal or
no harm to the patient. It may also focus on a new incident for which a comprehensive analysis
was recently completed. Other incident analysis tools may not lend themselves to use in a concise
approach, or be used in a limited way (e.g. sequence of events, constellation diagram, etc.).
Table 5. Characteristics of concise and comprehensive incident analysis
(52)
Characteristic Concise Comprehensive
Should include person(s) with knowledge of incident
analysis, human factors and eective solutions
development.
3 3
Oen conducted by an individual with input gathered
from the patient, family, sta and physicians local to the
incident as organisational or external experts.
3 8
Conducted by an inter-disciplinary medium to large ad
hoc group (may include patients, family members, sta
and clinician local to the incident as well as recognised
independent internal or external experts/ consultants not
involved in the incident).
8 3
Time taken for analysis
Short sequence of
events (hours to days)
Longer sequence of
events (up to 90 days)
Identies contributing factors as well as remedial
action(s) taken (if any)
3
(focus on key factors)
3
Recommendations for improvement
3
(if applicable)
3
Principles of incident analysis
Reflects the intent but
may not address all
Incorporates all
principles
Evaluation strategy
3
(if applicable)
3
Concise analysis is typically conducted by one person (analyser) with knowledge and skill in incident
analysis, human factors and eective solutions development. The facilitator usually gains this
expertise through a variety of formal education programs and mentored experience and practice.
The individual may be a healthcare clinician and/or other process expert, and not necessarily a risk
manager or patient safety/quality improvement ocer.

Step 4 – Analysis process | 53
Steps in conducting a concise
analysis
What happened
It is vital to obtain sucient information
to understand what happened in order to
understand how and why it happened. The
analyser may conduct informal discussions with
the patient/family/carer, healthcare clinician/s,
manager and/or expert/s in the process/es and
examine the equipment involved in the incident.
It is helpful to document key factual information
in the form of a high-level sequence of events or
narrative description.
How and why it
happened
1. Analyse the guiding questions to briefly
explore all categories, being mindful to
move away from patient-clinician interface
to systems level order to identify chains of
contributing factors (Appendix L).
2. Select some of the guiding questions or
develop unique incident specic questions
to informally discuss the incident with a few
individuals (this may include the patient,
family member, carer, sta and/or clinicians
local to the incident as well as organisational
or external experts).
3. A constellation diagram may be used
to facilitate a systematic approach. The
process of developing a constellation
diagram is intended to assist in the building
of a visual representation of the incident
and the system contributing factors. It is
also possible to identify mitigating factors
that prevented the incident from being
more signicant. See Appendix E for an
explanation of the constellation diagram.
4. Once all of the contributing factors have
been identied, it is appropriate to try to
understand how these factors are clustered/
linked with one another given that all
incidents generally result from a cascade of
events rather than an isolated contributing
factor.
5. Once the cluster/linkage is completed, it
is appropriate to transition to describing
the ndings and the development of
recommendations (if appropriate) to make
care safer for future patients in similar
circumstances.
6. Identify the key contributing factors that
contributed to the outcome by asking why
and how they are related.
What can be done
to reduce the risk of
recurrence and make
care safer?
Summarise the ndings and determine if there
is sucient data to develop recommended
actions. Are there known or easily identiable
evidence-based actions for improvement?
• If no, is there sucient knowledge and
expertise to develop local solutions for
testing, evaluation and formalization of the
response?
• If yes, proceed with formalising
recommended actions and consult with
relevant decision maker for decision
and action. See the following section for
additional information on developing and
managing recommended actions.
The analyser (or other person/s designated by
the organisation) formalises the action plan
and ensures that an evaluation strategy is in
place to determine if recommendations were
implemented and sustained, as well as if there
was any known impact to the safety of patients
within the targeted care process/es.
Determine if a multi-incident analysis is required
to eectively understand the applicable risks to
patients (see the following section).
Track and document all key decisions and the
action plan/evaluation strategy if applicable.

54 | Best practice guide to clinical incident management Second edition - January 2023
What was learned
Concise analysis can contribute important
knowledge regarding a larger number of
incidents and their contributing factors. The
general lessons should be disseminated
and ndings and/or recommended actions
should flow into the higher organisational
level for prioritisation of risks and actions for
improvement within the organisation.

Step 4 – Analysis process | 55
Multi-incident analysis
The benet of conducting a multi-incident
analysis is the potential to reveal patterns and
trends of contributing factors that are otherwise
not previously perceptible. These analyses can
also review previous recommendations and
identify those that were or were not eective.
For example:
• A group of individual patient safety
incidents, similar in composition and/or
origin, that caused no harm or lesser
degrees of harm.
• A group of individual patient safety
incidents, similar in composition and/or
origin, that may have caused varying degrees
of harm (no harm to catastrophic/major
harm).
• A group of patients who are impacted by
a similar contributing factor/s, and who
experience the same harmful incident (to
greater or lesser degrees).
• A group of completed comprehensive and/or
concise incident analyses.
For the purpose of this Guide, an analysis of
multiple incidents (or more than one) is called
multi-incident analysis. Alternate terms used
in the literature for this type of analysis include
cluster, aggregated and meta-analysis. Common
features of any multi-incident analysis include:
• pre-dened theme or scope
• involvement of an interdisciplinary team
including frontline clinicians and possibly a
patient representative
• use of quantitative and qualitative
methodologies.
Refer to Figure 10 for a multi-incident analysis
flow and pages 57 and 58 detail four examples.

56 | Best practice guide to clinical incident management Second edition - January 2023
Figure 10. Flow diagram for multi analysis
Follow through—implement, monitor, assess
Close the loop—share what was learned (internally and externally)
Develop and manage recommended actions
What can be done to reduce the risk of recurrence and make care safer?
• Develop recommended actions
• Suggest an order of priority
• Forward to applicable decision make for nal decisions and actions
• Timeframes for recommended actions
• Responsibility for recommended actions
• Evaluation criteria.
Determine how and why it happened
• Complete a qualitative analysis compare and contrast contributing factors and/or
recommended actions to look for common trends or themes
• Summarise ndings include any trends, patterns of contributing factors, and any other ndings.
Before the incident Immediate response Prepare for analysis
• Determine the theme and inclusion criteria
• Gather data
• Convene an interdisciplinary team
• Review the literature and obtain expert
opinions to lend perspective to the analysis
• Develop the analysis plan and prepare the materials.
Understand what happened
• Analyse incident reports and /or analysis and supporting information
• Analyse additional information policies, procedures, literature, environmental scan
• Previously reported incidents, previous analyses, consultations with colleagues or experts, etc.
• Compare and contrast the incident reports and /or analyses that comprise the theme
• Analysis (can use process mapping)
• Compete a quantitative analsyis (descriptive statistics).
Analysis process
MULTI-INCIDENT
Examples that describe various types of multi-incident analyses and the methodology for conducting
such analyses are provided next.

Step 4 – Analysis process | 57
Examples of multi-incident
analysis
Example 1: A group of low and no harm
incidents or near misses that have not been
analysed.
All healthcare organisations have reporting
systems in place to enable sta to report
incidents that may have caused no harm
or lesser degrees of harm. Although it is
generally agreed that these incidents are
valuable learning opportunities, in the
absence of signicant patient harm they
are frequently led away with little or no
analysis. In particular, when multiple no or
low harm incidents are analysed as a group,
they have the potential to reveal trends or
patterns of contributing factors that may
not be identiable by looking at a single
incident. If actions are identied and taken
as a result of this collated type of analysis,
future incidents might be avoided.
This type of analysis would include three
or more no harm, low harm and near miss
incidents that have not previously been
analysed as a part of a patient safety
incident analysis. For example, an analysis
of 15 falls or near falls that identied
common patterns of contributing factors
and safety deciencies was conducted by
Zecevic et al.
(56)
Example 2: A group of incidents that are
similar in composition and/or origin that
may have caused varying degrees of harm
(no harm to catastrophic/major harm).
Some healthcare organisations may decide
to analyse multiple incidents involving a
predened theme or criteria. The patient
outcome of these incidents may be varied—
from no harm to catastrophic/major harm.
For example, all falls occurring in an in-
patient acute care unit during a six month
period, including eight incidents that were
low harm and not analysed, and one event
where there was severe patient harm and
a comprehensive analysis was previously
conducted.
This type of analysis would include three
or more near miss, no harm, low harm, or
signicant harm incidents occurring within a
dened period of time or location. As noted
above, one or more of these may have been
previously analysed using a comprehensive
or concise analysis methodology.
Example 3: A group of patients who are
impacted by a similar contributing factor/s,
who experience the same harmful incidents
(to greater or lesser degrees).
The theme of this type of analysis is where
a common outcome may impact multiple
patients. Although the contributing
factors may be complex and unique to
each incident, learning can be achieved
by analysing these multi-patient incident
analyses, frailties in healthcare systems can
be revealed and improvement strategies
implemented.

58 | Best practice guide to clinical incident management Second edition - January 2023
Example 4: A group of completed
comprehensive and/or concise incident
analysis.
Organisations that conduct analysis of
individual patient safety incidents will
accumulate a rich source of information
regarding identied risks, contributing
factors and action plans to reduce these
risks for patients. Organisations are
encouraged to develop and utilise a
management system to coordinate the
learning and ensure what is learned about
the health system is not lost or forgotten.
An analysis of multiple comprehensive
and/or concise event analyses
(46,55,57)
is not
unlike an aggregate or epidemiologic meta-
analysis, although it does not have precise
scientic and statistical methodology
associated with it. This analysis consists of
a group of completed analyses conducted
on similar types of incidents.
Ideally an organisation will employ a
management system to coordinate the
identication of overarching themes
related to multiple incidents that have
been analysed. The overarching themes
may include types of incidents analysed,
contributing factors identied and action
plans to reduce harm to patients. For
instance, there may be a number of
recommended actions made by analysers
that identify the need for improved
teamwork and/or communication. This
may lead to the design of a strategic
improvement priority for the organisation
with designation of appropriate resources to
support the eort. Queensland Department
of Health and Patient Safety and Quality,
Clinical Excellence Queensland, share
their learnings through publishing learning
updates.
Steps in conducting a multi-
incident analysis
Prepare for analysis:
• Determine the theme and inclusion criteria
e.g. identify the characteristics of incidents
to be analysed (no harm to catastrophic
harm) or multi-patient incidents, or identify a
theme for multiple completed analyses to be
analysed.
• Gather applicable data:
- if applicable, conduct interviews with
clinicians, patients/families/carers and
others with knowledge of the incidents
and/or care processes involved in the
incidents.
• Analyse literature and obtain expert opinions
to collect additional background and
contextual information and lend perspective
to the analysis:
- analyse other reporting and learning
systems.
• Develop the analysis plan, which will include
both qualitative and quantitative analysis
elements.
What happened
Analyse the patient safety incidents and/or
previous comprehensive and concise analyses
to look for common trends, patterns and issues.
This will include comparing and contrasting
sequence of events, contributing factors, and
recommended actions from previous incident
analysis. Process mapping, a tool frequently
used to support Failure Mode and Eects
Analysis (FMEA)
(50,58)
and Lean improvement
methodology
(51)
can also be used to support
the identication of system weaknesses when
conducting an analysis of multiple incidents.
Note the frequency of system issues or failure
points and if applicable, recommended actions.
This represents the quantitative portion of the
analysis and will include classications such
as severity of harm type of incident, patient
diagnosis, etc.

Step 4 – Analysis process | 59
Handy tip
For all methods of analysis:
• Gather information:
− interviews
− brainstorming
− retrospective clinical records
− multidisciplinary team analysis
− photographs, diagrams or drawings.
• Map the incident:
− narrative sequence of events
− tabular sequence of events
− cause and eect diagram.
• Identify care and service delivery
problems:
− multidisciplinary analysis meeting
− brainstorming/brain writing
− nominal group technique
− change analysis.
• Analyse problems to identify
contributory factors and root causes:
− draw a diagram (e.g. constellation
diagram)
− contributory factors classication/
guide
− ve whys.
• Generate solutions and
recommendations:
− barrier analysis
− risk benet analysis.
How and why
it happened
The qualitative analysis involves focusing on
the identied contributing factors as well as
similarities that may not have been apparent
through an individual incident analysis.
Narrative descriptions are particularly helpful for
this portion of the analysis. As common patterns
are identied, the team may need to further sub-
categorise to clarify trends or issues.
When a group of comprehensive and/or concise
analyses are analysed both the contributing
factors and the recommended actions may be
included in the qualitative analysis.
Summarise ndings including contributing
factors and previously recommended actions
that may lead to system improvement. Include
any trends, patterns or contributing factors and
any other ndings.
What can be done
to reduce the risk of
recurrence and make
care safer?
Develop recommended actions that will lead
to system improvement, giving consideration
to available supporting information, including
evidence-based guidelines and leading
practices. Identify short-term and long-term
strategies. See the next section for guidance
in building eective recommended actions to
reduce risk.
It is helpful for the team to consider a
measurement and evaluation strategy before
forwarding recommended actions to applicable
decision makers for nal decisions and
delegation for implementation.
What was learned
The ndings (contributing factors, trends and
themes), recommended actions and their
outcomes should flow into and be coordinated
with the organisation’s risk management and
improvement processes, including processes
for communicating and sharing learnings.
See Appendix P (Lessons learned) for more
information.

60 | Best practice guide to clinical incident management Second edition - January 2023
Recommended actions
Develop and manage
recommended actions
Developing and managing recommended
actions involves a series of activities at
several levels of the organisation aimed to
determine what can be done to reduce the risk
of recurrence and make care safer. The success
of the recommended actions is dependent on
the quality of ndings identied in the previous
analysis step (how and why it happened).
It is important to consider that a few well
thought out high-leverage recommendations
will ultimately be more eective than a lengthy
list of low impact actions. Figure 11 illustrates
a hierarchy of eectiveness and leverage for
recommendations.
The Patient Safety Health Service
Directive Guideline for Clinical Incident
Management (QH-HSDGDL-032-3) states
that HHS “should have an established
local documented process for the
development of recommendations
arising from SAC1 analysis, that
should include engaging with
relevant stakeholders and prioritising
recommendations based on impact and
achievability”.
The analysis team has a fundamental role in
the development of recommended actions.
Findings identied in the previous analysis
step (how and why it happened) are analysed
by the team and actions proposed to address
the contributing factors that allowed the
incident to occur. Use of analysis diagrams
(like the constellation diagram) assists to
support teams in evaluating the best leverage
points for recommended actions. The analysis
team is generally responsible for proposing
recommended actions, suggesting an order
of priority, proposing timeframes, responsible
positions and consulting with others such as
treating clinicians before the analysis report is
handed o to those responsible for validating
and implementing the actions.
Handy tip
Recommendations made in conjunction
with the treating clinicians and the relevant
stakeholders, directly responsible for
implementing, are far more powerful and
likely to produce results than those that are
delivered to them by ‘people’ who have not
been consulted.
Note that in rare instances, analyses may
not generate any new recommended actions
(in particular, concise analysis).
Key features of eective
recommended actions
Healthcare leaders and those involved
in analysis in Queensland healthcare
organisations have expressed the need for
a tool to help build more robust and precise
recommended actions. The list of key features
presented below is a guide that can be
adapted by teams and used locally to focus on
developing eective recommendations/actions.
Eective recommended actions:
• Address the risk associated with the ndings
identied during the analysis.
• Utilise the most eective solutions
that are reasonable/possible given the
circumstances.
(52)
• Oer a long-term solution to the problem.
• Ensure they are formulated using the
SMARTER format
(59)
as in Table 6.
• Target the actions at the right level of the
system and ensure the action is appropriate
for that level (see Step 1 for a description
of system levels). For example, if one of
the recommendations from a medication
error is to change the label design, the
responsibility for implementation may
lie outside the organisation where the
incident occurred, requiring a national or
international eort.
• Assign responsibility at the appropriate level
in the organisation.

Step 4 – Analysis process | 61
• Ensure there is a greater positive than
negative impact on other processes,
resources and schedules (balancing
measures should be in place to ensure that
unintended consequences are known and
understood).
• Ensure recommendations are based on
evidence that demonstrates the impact of
this or similar action. Consider research
literature, similar recommendations
implemented in the organisation (e.g.
from accreditation, patient complaints) or
externally; and review patient safety alerts
and advisories. Aim to use the highest level
of evidence available (randomised controlled
Figure 11. Hierarchy of eectiveness
(61)
Rules and policies
(eg. policies to prohibit borrowing
doses from other areas)
Education and information
(eg. education sessions on
high-rish medicines)
Low leverage
Human-based System-based
Least eective
Simplication and
standardising
(eg. standardisation
paper or electronic
order sets)
Reminders,
checklists, double
checks
(eg. independent double
checks for high-risk
Medium leverage
Moderately eective
Forcing functions
and constraints
(eg. removal of a
product from use)
Automation or
computerisation
(eg. automated
patient-specic
despensing)
High leverage
Most eective
Most
eective
Less
eective
System
orientated
Human
orientated
Table 6. SMARTER format
(59)
Specic
Be clear about the issue you are targeting and write exactly what you recommend to
address it.
Measurable
How will you know when the goal has been accomplished? Aim to incorporate quantiable
indicators into regular monitoring and reporting cycles where possible.
Accountable/
Achievable
Who is going to ensure these are followed through? Allocate single point of accountability
for implementation of each recommendation. Can it be achieved with available resources?
Reasonable/
Realistic
Are the recommendations achievable given the current budget and available resources?
Timely
Prioritise your recommendations, streamline into manageable tasks for the implementing
teams and allocate a target date.
Eective
Will this recommendation make a dierence? Eective recommendations should reduce
the frequency of a future incident recurring.
Reviewed
There is merit in having an external person/body/committee review all recommendations
to assess whether they will achieve their intended outcome, and not negatively impact on
other areas.
trials are the highest, followed by controlled
observational studies, uncontrolled studies,
opinion of experts and opinion of peers).
(60,61)
• Provide enough context to ensure that if the
action is implemented, those responsible
will understand the rationale behind it.
• Consider whether there will be sucient
resources, engagement and willingness
to change in order to fully implement and
sustain the recommendations.
• Consider whether the recommendations
will need to be tested in Plan-Do-Check-
Act cycles, to learn what works and what
doesn’t, prior to full implementation.

62 | Best practice guide to clinical incident management Second edition - January 2023
Handy tip
What to consider when developing
recommendations:
• understand that re-training is not always
the right solution
• intelligent use of checklists, policies and
protocols
• minimal dependency on short-term
memory and attention span
• simplication of tasks and processes
• standardisation of tasks and processes
• avoidance of fatigue (analysis of working
hours/patterns)
• alignment with evidence-based practice
• alignment with organisational priorities
and risk registers.
One of the benets of using human factors
principles to assist in identifying contributing
factors is that the same approach can be used
to identify and evaluate the eectiveness
of recommended actions. In other words,
identifying systems-based contributing factors
correctly should lead to system-based solutions.
When recommending actions, many possible
categories of options with varying degrees of
eectiveness are available. The team should
appraise this range (see Figure 11, listed in order
from most eective to least eective) and be
encouraged to recommend the most eective
solution that is reasonable and/or possible
given the circumstances. Note that items
such as training and policy development are
necessary components, but when used alone,
do not change the underlying conditions that
lead to the incident.
From a human factors standpoint, the
strongest interventions are ‘physical rather
than procedural and permanent rather than
temporary.’
(52)
Organisations may nd the
assistance of human factors engineers or
ergonomists helpful in determining if the
proposed actions will be eective from a human
factors perspective.
In many cases, a system-based recommended
action involves a change or improvement to
a process or protocol, work areas, soware,
order forms or equipment. A mistake-
proong step during the development of
recommendations assists teams to determine
whether the recommended action/s will
have the desired eect/s. In this step, team
members assess whether the recommended
action, if implemented, would have prevented
the incident or mitigated the harm. It is also
an opportunity to consider the potential for
introducing unintended consequences to
processes (e.g. creating unnecessary steps or
added workload, possibly leading to unsafe
work arounds).
Consideration needs to be given to evaluating
the likely impact of the actions before
implementation. One way to do this is to
conduct one or more of the methods described
in Appendix M—cognitive walkthrough, heuristic
evaluation or usability testing. The method
selected will depend on the complexity of the
sub- system being changed and the potential
severity if the recommended action fails or
introduces unintended consequences. If the
potential failure or unintended consequence is
potentially more severe, it should be elevated
with usability testing or a combination of
the methods, and the recommended action
modied and improved before implementation.
FMEA
(50,58)
is another prospective analysis
technique that can be used to evaluate the
impact of a proposed process change.
The initial focus is on the elimination of risk
to patients. If there are no actions that can be
applied to eliminate the risk, the team should
seek the most appropriate controls to reduce
the possibility of recurrence. It is important
to note that applying a control means that
although checks will be in place, there still is
a chance of reproducing the same or related
circumstances that led to the original incident.
There are occasionally circumstances under
which a team may choose to accept that one or
more identied factors cannot be altered. For
example, in analysing an incident related to lack
of timely access to tertiary care, the team would
have to accept the fact that this level of service
will not be made available in remote locations
and focus attention on rapid transfer of patients
when such services are needed (in other words,
implement a control measure).

Step 4 – Analysis process | 63
Handy tip
A few well thought out high impact
recommendations will ultimately be more
eective than a lengthy list of low impact
actions.
Suggest an order of priority for
recommended actions
The need to prioritise the recommended actions
is the result of several practical factors:
(62,63)
• Related to the organisation:
− abundance of recommendations
from multiple sources generated from
accreditation, patient complaints,
insurance claims, coroner reports and
other
− limited resources (budget, sta time) to
ensure good follow through of quality
improvement and risk management
initiatives
− additional priorities and strategies
described in strategic plans.
• Related to the external environment:
− a variety of external pressure and
requirements influence operations
including required organisational
practices, regulatory and policy
requirements
− public reporting and compliance with
certain indicators
− reports of similar incidents publicly
available.
• Related to the characteristics of the
recommended action itself (degree of
change required).
The analysis team is generally responsible
for suggesting an order of priority and
desired sequence of events for completion of
recommended actions. This is later conrmed
by the executive team and delegated for
implementation. The following criteria may
assist in the prioritisation process:
• If the recommended action is not
implemented, what are the risks (the worst
possible outcome) for the patient, clinicians,
organisation? If possible, rate the risk using
the consequence and likelihood assessment
as in Table 7.
• Which actions can be immediately
implemented? Consider if there are quick,
safe patient care wins that will empower the
implementation team and others to continue
(it is important to emphasise small wins
are steps in the right direction, not the nal
destination).
• Consider if there are existing mechanisms
(initiatives, programs or other improvement
eorts) in place to implement the
recommended action/s. Building an
inventory (via a table, spreadsheet or other
register) of current eorts to address this
or similar issues (contributing factors)
can prove valuable for improvement. The
searchable inventory could be a living
document maintained and used by all levels
in the organisation.
• If possible:
− recommend actions for dierent levels in
the organisations and discuss what the
most important action is at each level.
− estimate the resources (human
and nancial) and sequence of
events needed to implement each
recommended action.
Table 7. Risk assessment matrix
(64)
Consequence
Negligible Minor Moderate Major Extreme
Likelihood
Almost certain Medium (7) Medium (11) High (17) Very High (23) Very High (25)
Likely Medium (6) Medium (10) High (16) High (20) Very High (24)
Possible Low (3) Medium (9) High (15) High (18) High (22)
Unlikely Low (2) Medium (8) Medium (12) Medium (14) High (21)
Rare Low (1) Low (4) Low (5) Medium (13) High (19)

64 | Best practice guide to clinical incident management Second edition - January 2023
An example of a tool that can be used to summarise the dra prioritised recommended actions is
provided in Table 8. For each column, enter a descriptor (high/medium/low or other as applicable), or
a few short comments
Table 8. Example of table to summarise and prioritise recommended actions
Recommendation/s summary
Risk
(high,
medium,
low)
Hierarchy of
eectiveness
(high, medium,
low leverage)
Predictors
of success
System level
targeted
(micro, meso,
macro, mega)
Note if
evidence is
available and
what type
Conrm
validity,
feasibility
(high,
medium,
low)
Order of
priority/
timeframe
Handy tip
Recommendations should:
• be clearly linked to identied
contributing factors or key learning
point/s (to address the problems rather
than the symptoms)
• be designed to signicantly reduce the
likelihood of recurrence and/or severity
of outcome
• be clear and concise and kept to a
minimum wherever possible
• be specic, measurable, achievable,
realistic, timely, eective and reviewed
(SMARTER) so that changes and
improvements can be evaluated
• be prioritised wherever possible
• be categorised as those:
− specic to the area where the
incident happened
− that are common only to the
organisation involved
− that are universal to all and, as such,
have statewide or even national
signicance.
Recommendations might also include
provision of ongoing support of patients
and sta aected by the incident.
Strength of recommendation/s
To design strong recommendations, aim for high
impact, low eort interventions such as forcing
functions, architectural re-design, soware
changes, standardise and simplify processes.
The stronger the action, the more likely that it
will work. The weaker the action, such as writing
policy or training sta, the less likely it will be
sucient to prevent a similar clinical incident
occurring again. For training to hold its value it
needs to be recurrent; nevertheless it remains a
weak action.
Table 9 provides a comparison of strength of
actions and the related eort required over time
to implement.

Step 4 – Analysis process | 65
Table 9. Strength of recommendations eect and eort actions
Strength of recommendation
Strong actions Moderate actions Weaker actions
When implemented strong actions
rely less on people’s actions.
Most likely to be eective and
sustainable.
Systems x. Remains reliant on
individuals/ team vigilance.
Reliance on the individual.
Rely on human behaviour.
Less likely to be eective /
sustainable.
• Architectural/physical plant
changes
• Tangible involvement & action
by leadership in support of
patient safety
• Simplify the process and
remove unnecessary steps
• Standardise equipment or
process or care maps
• New device with usability
testing before purchasing
• Checklist/cognitive aid
• Increase in stang/decrease
in workload
• Read back process
• Audit
• Enhanced documentation/
communication
• Soware enhancements?
Modications
• Simulation based training
• Eliminate look and sound-a-
likes
• Eliminate/reduce distractions
(sterile medical environment)
• Redundancy/double checks
Warnings and labels
• New procedure/
memorandum/policy
• Training
• Additional study/analysis
Eort to implement recommendations
High Moderate Low
>12 months 6-9 months 3-6 months
Consult on the dra
recommended actions
Where possible, a consultation step may
be benecial in order to ensure that the
recommendations are appropriate, the
identied risks have been addressed, and there
is a high probability to reduce the recurrence
of this or similar incidents. Patients/families/
carers have a unique perspective on the
incident and should be invited to provide their
improvement ideas to the team. Clinicians
from the area where the incident occurred,
as well as experts should also be consulted.
Those providing feedback on potential actions
should be advised that their suggestions will be
considered, but may for a number of reasons,
not be implemented. These reasons should be
explained to the contributor.
It may be appropriate again at this time to
reprioritise the recommendations with the use
of an Impact and Achievability Matrix (see Figure
12) to determine both the achievability of your
recommendations (i.e. what is within the control
and the means of the health service) and also
the impact of the recommendations (i.e. on the
prevention of the patient harm that has led to
this analysis). Using this tool assists in reaching
consensus among the team on which of the
recommendations are best to prioritise your
eorts and resources on.
Figure 12. Impact and Achievability Matrix
ACHIEVABILITY
IMPACT
Low impact High impact
Inside your controlOutside your control

66 | Best practice guide to clinical incident management Second edition - January 2023
Prepare and hand-over report
A nal task of the analysis team is to include
the recommended actions and the ndings of
the analysis in a report that is provided to those
responsible for:
• approving the actions
• delegating them for implementation
• allocating resources
• empowering and monitoring implementation
(most frequently an executive manager or
quality committee).
Having a clear record of the analysis and
relevant supporting documentation will support
condence in decisions related to the analysis.
If the steps, facts, evidence and supporting
documentation are tracked throughout the
analysis, the writing of the report should be
relatively straightforward. It is suggested that
the report have headings and sub-headings,
as it will inform the basis for those responsible
to make decisions regarding recommended
actions.
Frequently, the analysis team will disband once
the report is handed over for implementation.
To ensure appropriate follow-up, a tracking
mechanism should be put in place to trace the
implementation of recommended actions and
their accompanying outcomes (see Table 11 for
an example).
Manage recommended actions
The individual or group of individuals (likely
an executive manager or organisational quality
committee) receiving the analysis report are
responsible to ensure that the recommended
actions are validated from a strategic and
operational perspective, as well as delegated
appropriately to implement the approved
actions. This individual or group of individuals
will generally be required to support decisions
related to the implementation of actions to
organisational leaders and other stakeholders,
while demonstrating good stewardship of
available resources and considering the long-
term well-being of the organisation.
Validate actions from strategic
and operational perspectives
The analysis report, including recommended
actions, needs to be evaluated by the
responsible individual/s in order to decide if
and how actions should be implemented. The
following three steps may be helpful in guiding
their decisions:
1. Conrm actions
To facilitate conrmation of the
recommended actions, the responsible
individual/s may choose to begin by
merging actions from the analysis with
recommendations from other sources.
This builds on the inventory generated by
the analysis team (Table 10) and aims to
ensure that actions are considered in light of
strategic and operational risks and priorities.
Ideally, a centralised inventory is created
to capture current recommendations in
the organisation from all sources and their
status (e.g. patient complaints, trigger tool
ndings, insurance claims, accreditation,
coroner). The inventory can be housed in
a simple spreadsheet or included in the
organisation’s patient safety or performance
systems.
It may be helpful to consider sorting
the recommended actions by the main
categories of contributing factors (e.g. task,
equipment, work environment, patient, care
team, organisation, other) and including
high level key information about each
recommended action (e.g. estimated risk for
the organisation, implementation status).
An inventory will assist with the prioritisation
steps by ensuring the recommended actions
for this incident are aligned with, and not
competing with other ongoing eorts, in the
organisation. Regular maintenance of such
an inventory is required.

Step 4 – Analysis process | 67
2. Assess validity
Validating the recommended actions is
important and will ensure that the actions are:
• attainable (the resources, competence
and tools needed are available—if not,
there is a plan to put them in place before
implementation starts)
• feasible (the culture, readiness for
change, technology, legislation and other
contextual factors support the action and
are not completing with it)
• cost eective (potentially a cost benet
analysis may be needed)
• aligned with the strategic and
operational priorities of the organisation
(implementation of the actions will not
create a void in other areas or programs).
3. Approve and set guidelines for implementation
A nal validation step includes conrmation of
the actions to be implemented and high-level
guidelines for implementation. Guidelines
for implementation should focus around the
following criteria and include a brief rationale:
• Set an order of priority for the actions—
what should be implemented rst?
• Specify the system level targeted (micro,
meso, macro or mega). Consider if
the recommended actions should be
generalised to other areas of the system.
For example, if the incident is related
to the use of a concentrated form of an
injectable medication in one area of a
hospital, it would be benecial to address
the management of the medication in
all areas of the hospital and consider
the management of similar concentrated
injectable medications using the same
intervention, at the same time.
• Sequence of events—start time and
estimated duration.
• Accountability—include a senior leader
and an implementation lead.
• Propose success measures, milestones
and determine reporting frequency.
Once approved and validated, recommended
actions are prepared for hand-o to the team and
individual/s responsible for implementation.
There should be a process in place to share
information about actions recommended and
implemented with the patient and family as
well as with the clinicians in the area where the
incident occurred, organisational leaders, and
others as needed. Step 6 provides for more
information about learning and sharing.
Delegate recommended actions
for implementation and empower
implementation
The approved recommended actions are handed
over to the team or individual/s responsible to
implement the action. If possible, this should be
done during an in-person meeting, so everyone
has a common understanding and is clear on the
purpose, objectives and direction of the actions.
Clarity is important because the senior leader
and the team responsible for implementation
will base their work plans on the information
received about the recommended actions during
the hand-o process. It is important to ensure
follow-through and follow-up of the status of the
actions.
Translating incident analysis recommendations
into action and sustainable change is not easy.
See Table 10 as a tool to track implementation
and status of recommendations.
Table 10. Example of a tool to track the implementation status of recommended actions
Implementation status
Category
Recommended
action
Expected
completion date
Source
and ID#
Date
entered
Progress
status
Priority /
Timeframe
Risk
level
Responsible
person
Task
factors
Real improvement will only occur when a systematic, collaborative approach is
used that has explicit leadership support and sucient resources. These resources
must include quality improvement and patient safety facilitators who have received
ongoing education in the applicable methodologies and have developed and honed
their skills over many years of experience.
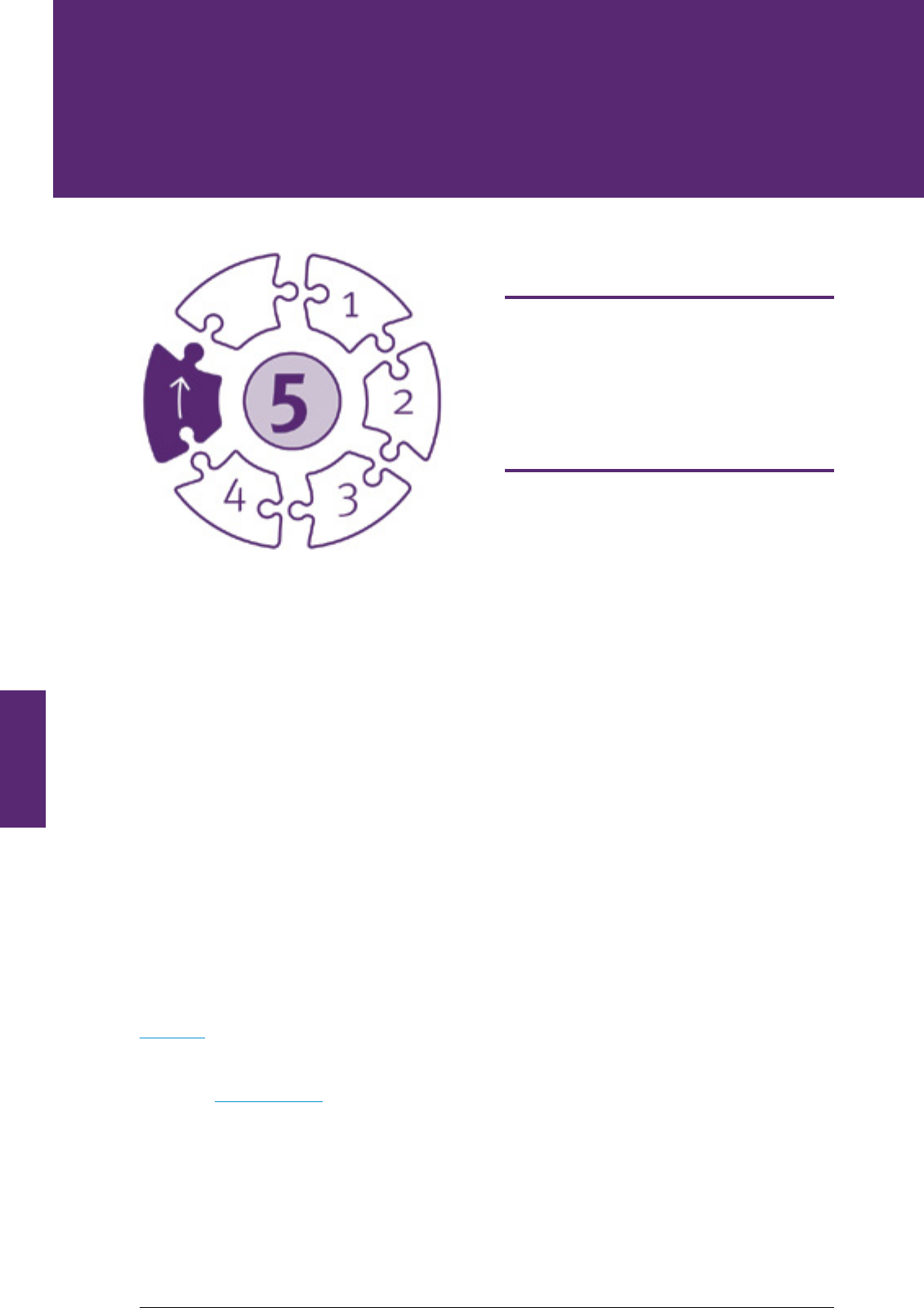
68 | Best practice guide to clinical incident management Second edition - January 2023
Implement recommended actions
The implementation of recommended actions is
an important step in the incident management
process, with its eectiveness contributing
to the overall success of the incident analysis
process. Board directors and executive leaders
have key roles to play in monitoring clinical
incident management performance, in particular
enacting the governance and ensuring
legislation requirements are met, considering
the eectiveness of potential recommendations
and ensuring the sustainability of strategies.
The Executive (senior leadership), therefore
have the additional responsibility of providing
direct guidance in the process of developing
recommendations. This involvement can
accelerate the implementation of supported
recommendations and promote a culture of
safety in the organisation. There are governance
resources (eg Fact sheet: Key Information for
Board Directors and Executives) available for
Board members that oer valuable support for
fostering a culture of safety.
Implementation can be very challenging if the
actions are not focused on the contributing
factors, do not have clear objectives, are
not communicated clearly or are not visibly
supported by the senior team. In addition
to this, capacity to take on new initiatives in
healthcare is challenging at times—frontline
teams are always busy caring for patients and
implementing current improvement eorts, and
managers may feel time poor with additional
change based projects that may be added to
their usual day-to-day operations. To add to
these existing pressures, it is expected that all
approved recommended actions from clinical
incident analyses will be implemented in a
timely manner.
To successfully implement recommendations,
especially the more challenging ones, an
appreciation of the key elements for improving a
system, helps with this understanding.
(65)
These elements are listed as follows:
1. Appreciation of the system: obtain a deep
understanding of the what and the why of
the underlying system. The problem can’t
be xed unless the problem is properly
understood.
2. Theory of knowledge: gain knowledge by
testing improvement recommendations to
see if they work. Apply small scale testing
and analysis using quality improvement
methodology if appropriate e.g. Plan-
Do-Study-Act (PDSA) cycles are oen
needed before attempting a full-scale
implementation.
– Implement recommended
actions
– Monitor and assess the
eectiveness of actions
Step 5: Follow through

Step 5 – Follow through | 69
3. Psychology: to implement change
successfully, it is important to understand
any underlying human factors. This is
important when trying to implement change
and to ensure sta are supported throughout
the change. It is also integral in designing
change initiatives and considering how
to overcome identied obstacles, such
as shortcomings in human memory and
attention.
4. Understanding data and data variation:
the use of data is required to evidence that
the implementation has been successful.
The data needs to be accessed from a valid
source, and include metrics based on well
understood numerators and denominators.
Any changes in the data need to be assessed
to see if they are real changes (hopefully
as a result of the implementation of your
recommendations: this is called special cause
variation) or whether the change is due to the
natural small scale variation that happen in all
processes (natural cause variation).
It is important to consider “work-as-
done” versus “work-as-imagined”.
“Work-as-imagined” describes what
should happen under normal working
conditions. Unfortunately, it does
not take account of sta constantly
adjusting to the complexity of
healthcare and the ever-changing
conditions of their work environment.
In contrast, “Work-as-done” describes
what actually happens and how
people deliver care, in the complex
reality of health services. Unless your
recommendations are implemented
in the “work-as-done” reality on the
clinical floor, your improvement eorts
are likely to be misdirected.
(34,66)
Complexity science suggests trying multiple
approaches and shiing time and attention to
those strategies that appear to be eective.
The PDSA process of small cycles of change
to implement quality improvements is
one example of an activity that enables
experimentation within a scientic approach.
(67)
The organisation should also consider piloting
or usability testing of interventions prior to
broad implementation, especially in situations
where substantial changes in process are
planned.
Successful implementation requires that senior
leaders have conrmed all the following:
• a “will” in the health service for the
change/s
• sucient executive support for the change/s
• sucient resources being made available for
the change/s
• an agreement/plan with executive sponsor
what you will do if there are barriers
• the team involved in implementation have
the necessary leadership skills, credibility,
communications ability, authority, analytical
skills and a sense of urgency.
Another easy to use and tested tool developed
by the Boston Consulting Group—DICE— can
assist with identifying and minimising the risk
of implementation failure.
(68)
Their experts
have determined that the outcome of change
initiatives is driven by four elements (DICE).
• the Duration of the project
• the performance Integrity of the team
• the organisational Commitment to change
and
• the additional Eort required of sta
members.
Ideally, implementers will share the progress
of their eorts with members of the analysis
team and the unit/ program/organisation
where the incident originally occurred. Once
implementation is complete, the results of the
evaluation and learning should be shared with
others.

70 | Best practice guide to clinical incident management Second edition - January 2023
Monitor and assess the
eectiveness of recommended
actions
The purpose of implementing system changes
is to make the system safer. However, some
recommended actions—even well intentioned
and well thought out changes—may not have
the desired eect in practice. As a result, the
eectiveness of the implemented recommended
actions must be monitored to determine if
the changes helped make the system safer,
had no or limited impact on the safety of the
system, or in the worst-case scenario, the
changes actually made the system less safe.
If surveillance indicates that, for whatever
reason, the changes did not have the intended
eect, the organisation needs to revisit the
recommended actions to identify alternative
solutions or to improve the impact of earlier
solutions. Organisations invest considerable
resources in investigating incidents in order to
alter the conditions which led to these events.
Monitoring the impact of recommended actions
of an incident analysis promotes organisational
learning and sta commitment to improve care.
Monitoring the eectiveness of recommended
actions requires the diligent use of
measurement approaches. One way to identify
useful measures is to ask sta how they would
know if an action was eective. Sta may be
more familiar with existing data or have ideas
about how to observe and record actions that
the analysis team may not recognise.
(52)
Data
that is available from existing data bases or
reports can be useful, as well as data that can
be recorded with simple audit tools used on
a regular basis. The most useful measures of
recommended actions are those that assess
outcomes. Outcome measures provide
direct evidence of the eectiveness of the
actions taken and not just the completion of
preventative measures.
Outcome measures should be complemented
with process measures that assess the extent to
which recommended actions are implemented.
A balance of outcome and process measures
allows the individual or group charged with
monitoring the recommended actions to
interpret their impact and to revise or reinforce
them if they fail to have the desired impact.
Evaluation or measurement?
The methodology involved in
evaluation is more complex than the
one for measurement because its
intent is larger: to make judgements,
improve or further develop (program)
eectiveness, inform decisions
and/or increase understanding.
(69)
Measurement is one of many
components in evaluation and quality
improvement.
Many incidents are rare, so monitoring weekly
or monthly incidence is not informative. In this
case more advanced strategies such as control
charts that monitor time between incidents
(70)
can be used. In settings where control charts
are not available, teams can use measures of
processes that identify important preventative
measures as substitutes or proxies for
outcomes.
Process measures should be displayed in
run charts to permit quick assessment of
performance over time. Run charts have
several advantages—they are easy to create
without specialised soware, they are
straightforward to interpret and they provide
more information than bar charts or tables
that do not show performance over time (and
can hide undesirable patterns of performance
including short term improvements that may not
be sustained).
(71)
Annotated run charts include
notes that help in understanding the factors that
contributed to the change in performance (see
example below in Figure 13). Run charts
are even more useful if they are interpreted
using a series of rules that signify non-random
patterns.
(72,73)

Step 5 – Follow through | 71
Figure 13. Run chart
X axis label
1 2 3 4 5
Goal
Y axis label
0
2
4
6
8
10
12
14
Median Value
The principal goal of measurement in
monitoring recommended actions is to assess
the improvement potential.
(74)
Measuring for
improvement emphasises a practical approach
with ‘just enough’ data in small sequential
samples.
(75)
Small samples taken frequently
can be more informative than large samples
taken less oen (and are also easier to
incorporate into sta work). Measures need
to be clearly dened and the strategies for
collecting data need to be developed with
the sta who will collect them. Collecting
baseline data on a process before changes
are introduced, is helpful in demonstrating
whether the changes are improvements and
are sustained over time. The sampling strategy
and timeframe for measurement must be
clearly stated. It is important to set realistic
performance thresholds (e.g. a target for 100
per cent compliance should not be set unless
it can be met).
Measurement may take the form of voluntary
reporting, intervention tracking, direct
observation of performance, chart review,
computerised tracking and surveys. Regardless,
it is important that measures be carefully
dened, that data collection be designed to
be practical and that sta are provided with
information on why measurement is important
and how it can be incorporated into their work.
Table 11 provides key questions in designing a
strategy to collect data.
Measurement sometimes looks like ‘just
more work’ and measurement that is not well
designed, incomplete or hastily done will not
be informative. Good measurement helps to
assure that improvements are made to ensure
safer care environments and can translate into
better outcomes for patients and more eective
working environments.
Table 11. Key questions in designing data collection
1. Have I dened the data so that I get
exactly what I want?
2. How accurate is it and does it matter?
3. How can the data help me?
4. Can I rely on it being consistent?
5. What will I do with the data?
6. Does my collection strategy work?
7. How will I display the data

72 | Best practice guide to clinical incident management Second edition - January 2023
Close the loop
Sharing what was learned is the ultimate
objective of clinical incident analysis and
is represented as the last element of the
continuum in the Guide and aims to close
the loop. Sharing the learnings both within
the organisation (with patients, families and
carers, those involved in the incident, the
analysis team, the executive leadership and
Board, Quality Assurance Committees, Clinical
Networks, Strategic Advisory Groups and others
as needed) and outside the organisation (social
media, conferences, webinars, podcasts) is
key to preventing additional harm and making
care safer. Without learning and sharing, the
patients and organisation are still vulnerable,
as the same or similar incidents could happen
again. Additionally, there is an opportunity
lost for other organisations beneting from the
learnings. Results of analyses should rollup into
organisation-wide reporting and be shared with
the senior leadership, Board and the public.
The incident management process needs to
be continuously monitored to ensure that it
is eective and reliable. Single-loop learning
involves changing methods and improving
eciency to obtain established objectives.
Consistent monitoring may also help to identify
areas for further improvement, this is known as
double-loop learning.
Double-loop learning is dierent from single
loop learning which focuses on ‘doing things
right’. Double loop learning focuses on
changing the objectives themselves (i.e. doing
the right things). This learning approach may
involve questioning the assumptions about
the improvement objective and rethinking new
alternatives, objectives, and ways to approach
the problem.
(76)
Continuous organisational
learning and sharing results
Learning from an incident, understanding
and articulating what can be done to prevent
its recurrence and heal relationships are the
ultimate goals of the patient safety incident
management process. It is of utmost importance
that the learning is fed backwards and forwards
through multiple communication channels.
Organisations may wish to conduct a multi-
incident analysis of several completed incident
analyses where similar incidents can be re-
examined to draw larger scale conclusions.
Feedback loops must be created for each
incident analysis to share the learning with the
various individuals and groups who assisted
with analysis and implementation activities.
The patient, family, carer and clinicians in the
service area where the incident occurred should
be informed and involved about what changes
– Share what was learned
(internally and externally)
Step 6: Close the loop

Step 6 – Close the loop | 73
have been implemented and with what results.
The incident analysis team will want to know
which of the contributing factors they identied
were acted upon. Likewise, the implementation
team will want to know which of the changes
(actions) they implemented had the greatest
impact.
This information may be shared in multiple
ways, including memoranda, storytelling,
huddles or any other modality the organisation
is uses for communicating. The need for timely
communication is an aspect that cannot be
overlooked. Individuals should be specically
assigned this important task so that it is
completed in a timely manner.
Feed-forward communication loops where
the learning is shared externally are just as
important because the same or similar incidents
can occur in any organisation, system or country
Reflecting on and improving
the quality of analysis and
management processes
Organisations are encouraged to periodically
dedicate time and resources to review and
evaluate how well the incident analysis and
incident management processes function within
their health services. The purpose of this review
eort is to ensure the processes are appropriate,
reliable, there is appropriate resources, and sta
strive to improve care. In addition, the review can
assist in developing and/or improving protocols,
checklists and other resources that help teams
manage incidents.
Factors that influence the quality of analysis
include:
(77)
• timeliness of completing the analysis
• quality and strength of recommended actions
• implementation of recommended actions
(completion status)
• eectiveness of the actions implemented in
reducing the recurrence of harm (monitoring)
• sharing what was learned (internal and
external)
• presence of one or more eective mitigating
factors (barriers)
• clinician’s perception of care safety.
Non-monetary incentives (e.g. awards)
(78)
that recognise those teams that demonstrate
Table 12. Patient Safety Notications (Alerts, Notices, Communiques)
N
A Patient Safety Alert is issued for urgent dissemination of information to Hospital and
Health Services about a patient safety matter needing immediate attention and action. It will
specify mandatory action/s to be taken by health services, assign responsibility for action
and the timeframes in which such actions should occur.
N
A Patient Safety Notice is issued to inform Hospital and Health Services about potential
quality and safety issues requiring a risk assessment at the local level to determine
appropriate action/s regarding any identied issues. The Patient Safety Notice will specify
that health services must undertake a risk assessment and recommends action to be taken.
N
A Patient Safety Communiqué is issued to disseminate quality and safety information to
Hospital and Health Services and Divisions to ensure lessons learnt from local, statewide,
national and international sources are shared across the health system in a proactive
manner.
and the learning from one organisation should be
transmitted to others to prevent harm. External
communication should include what happened,
why, what was the organisation’s response, what
actions (or changes) were implemented, and with
what results.
Alerts, advisories or communiques are common
tools for feed-forward communication. Sharing
de-identied learning with others (in a manner
that complies with privacy legislation) is highly
recommended to prevent similar harm and also to
help others with incident prevention management.
For example, patient safety notifications are
developed from reported incidents by the Patient
Safety and Quality, Clinical Excellence Queensland,
to share learnings across Queensland (see Table
12). Patient safety alerts and advisories may also
be accessed from national and international data
portals and may also be relevant to Queensland
clinicians.

74 | Best practice guide to clinical incident management Second edition - January 2023
improved performance can have a signicant
role in increasing engagement in the process
and therefore also work to improve the quality
of the analysis. The quality of the incident
analysis is extremely important in restoring
trust and rebuilding relationships amongst all
those involved in an incident and in building a
restorative just culture in the organisation.
Conclusion
Planning for, reviewing and improving the
safety of patient care is a fundamental aspect
of providing consistently delivered quality
healthcare services. This Guide oers advice
across the spectrum of clinical incident
management, including a range of approaches
to improve the safety of care practices in
healthcare organisations. It can positively
assist care providers to perform a system-
based analysis of patient safety incidents,
within the setting of a restorative just culture,
that includes the identication of contributory
factors, determination of recommended actions
to reduce risk, development of action plans,
and measurement strategies to evaluate the
eectiveness of the plan.
Striving to identify and address the underlying
reasons why incidents occur will lead to
a greater understanding of hazards in the
system and, ultimately, as we work to close the
loop, to a safer healthcare system for all. It is
integral that the culture of the entire healthcare
organisation move from a backward-looking
determination of blame to a focus on learning
and support for all people aected by the
clinical incident; a restorative just culture.

Appendices

76 | Best practice guide to clinical incident management Second edition - January 2023
List of gures
Figure E.1: Ishikawa (shbone) diagram 84
Figure E.2: Tree diagram 84
Figure E.3: Describe the incident 85
Figure E.4: Add contributing factors 85
Figure E.5: Dene relationships between potential contributing factors 86
Figure E.6: Dene relationships between potential contributing factors 87
Figure E.7: Completed constellation diagram 88
Figure K.1: Constellation diagram of abscond incident 99
Figure O.1: Constellation diagram of medication incident 114
List of tables
Table B.1: Reportable events 80
Table K.1: Detailed sequence of events for abscond incident 97
Table O.1: What happened: medication incident—nal sequence of events 112

Appendix A | 77
Appendix A
Analysis team membership, roles and responsibilities
Leader: someone knowledgeable about the general type of incident and has organisational authority
to implement the process.
Attributes:
• has strong analytical and clinical skills in the subject area.
Responsibilities:
• keeps team focused on incident
• provides support for cultural change
• supports team members in their analysis
• removes barriers faced by team members.
Facilitator: quality specialist, patient safety ocer or risk manager with knowledge and self-
condence.
Attributes:
• expertise in analytical methods and techniques
• skilled at group dynamics
• skilled at delegation
• skilled at group consensus building.
Responsibilities:
• coordinates team meetings
• keeps team focused on event
• facilitates constructive dialogue
• monitors sequence of events
• ensures that analysis process is followed per organisational protocol
• may be responsible for ensuring completion of nal report.
Individuals knowledgeable about subject area: depending on the type of incident, this will vary.
Clinical and non-clinical sta provide valuable insight. For instance, teams for suicide incidents
may include physical plant or architecture sta, case managers, medical ocers, nurses, security
personnel, etc. Teams analysing medication events may include pharmacists, biomedical
engineers, information technologists, medical ocers, nurses, administration sta, pharmacy
technicians, etc. Teams for patient falls may include physiotherapists, rehabilitation sta, medical
ocers and nurses, etc.
Attributes:
• extensive knowledge of the subject area
• credibility within organisation
• analytical, open minded
• interested.
Responsibilities:
• provide information relevant to the dierent steps involved in the incident
• provide information on the usual process
• help identify contributing factors and actions relevant to current practice.

78 | Best practice guide to clinical incident management Second edition - January 2023
Patient/family/carer or consumer representative
Attributes:
• understanding of the incident from a perspective dierent from others in the team
• ability to communicate their perspective and understanding of the incidents.
Responsibilities:
• provide their opinion, knowledge of the incident and other information to facilitate the
identication of what happened, how and why it happened, and what can be done to prevent
recurrence
• participation in constructive dialogue.
Senior leadership
Attributes:
• authority for decision making
• drives the safety culture by example.
Responsibilities:
• ensures that actions are implemented once approved
• ensures that sta are scheduled away from normal duty to participate in analysis
• ensure that results of analysis are communicated broadly
• ensure that healthcare clinicians and patient/family or representative involved are supported.
Other sta or consultants: include outside agencies as appropriate (home care, vendors, e tc.) as they
can provide information that is not available to members inside the organisation.
Attributes:
• specic knowledge of equipment, technology, etc. that may have contributed to event or may be
required for actions.
Responsibilities:
• provide expert opinion and knowledge to facilitate identication of contributing factors and/or
development of recommended actions.

Appendix B | 79
Appendix B
Incident reporting and investigation legislation
The information below is an overview only. Always refer to the relevant documents or legislation for
further information and obtain legal advice if necessary.
The following requirements for reporting Severity Assessment Code (SAC) 1* clinical incidents is
required by the Health Service Directive, Patient Safety which was issued by the Director-General
under section 47 of the HHB Act:
• Hospital and Health Services will report all SAC1 incidents to the Patient Safety and Quality, Clinical
Excellence Queensland by recording the incident in RiskMan within one business day of becoming
aware of the SAC1 event.
• Hospital and Health Services will conduct an analysis of all SAC1 incidents.
• Hospital and Health Services will submit a SAC1 analysis report to the Patient Safety and Quality,
Clinical Excellence Queensland within 90 calendar days of the incident being reported in RiskMan
as a SAC1 event.
• Each SAC1 analysis report must contain:
− a factual description of the event
− the factors identied as having contributed to the event
− recommendations to prevent or reduce the likelihood of a similar event happening again.
Please note, the following requirement of the HHB Act, (section 100(3)) if the SAC1 analysis report to be
submitted to Patient Safety and Quality, Clinical Excellence Queensland is a RCA report in accordance
with the Act, the report must not contain the name or address of the patient, the sta involved in
providing the health service or members of the RCA team.
*SAC1 incidents are those incidents resulting in death or likely permanent harm which is not
reasonably expected as an outcome of healthcare.
There is no legislation or binding policy in Queensland that prescribes which form of analysis
HHSs must undertake for SAC1 events. HHSs should be aware of the following enabling provisions
of the HHB Act, for some forms of analysis (e.g. RCA or clinical review) in deciding which form of
analysis to undertake for each SAC1 incident. Consideration should also be given to other forms of
analysis other than the legislation.
Section 123 and a reportable event
Where an RCA team is appointed under section 98 of the HHB Act, and it transpires that the event
is not a reportable event, the provisions in Part 6, Division 2 of that Act dealing with RCAs, will
apply as if the event were a reportable event (refer to section 123). This includes the provisions
dealing with disclosure of information and certain statutory protections. As an example, where
an RCA team has been appointed under section 98 for an incident which has been registered
as a SAC1, and the incident is then reassessed and rated as a SAC2, the statutory requirements,
obligations and protections in Part 6, Division 2 of the HHB Act (Root cause analysis) will continue
to apply, regardless of the SAC rating.
Root cause analysis
The HHB Act facilitates—but does not mandate—the use of RCA as a quality improvement
technique by providing protections from RCA reports and documents being used in legal
proceedings and providing certain protections for members of RCA teams (see sections 116 to 122).
For an RCA to attract the privileges and protections under the legislation it must (among other things)
meet the following summarised criteria:
• the SAC1 event to be analysed is a ‘reportable event’ as dened in the Hospital and Health Boards
Regulation 2012 (Figure B.1) or under section 123 noted above.

80 | Best practice guide to clinical incident management Second edition - January 2023
• a systematic process of analysis is applied which meets the requirements of section 100(1) of the
HHB Act such as:
– factors that contributed to the happening of the event may be identied
– remedial measures that could be implemented to prevent a recurrence of a similar event may be
identied.
• the analysis must not include:
– investigating the professional competence of a person in relation to the event
– nding out who is to blame for the happening of the event (refer to sections 102 and 103 HHB Act).
• the RCA team must comprise at least two persons and they must (refer to section 99(1) ) HHB Act:
– have the appropriate skills, knowledge and experience to conduct an RCA of the event, having
regard to the nature of the event
– have not been directly involved in providing the health service to be analysed.
Table B.1: Reportable events
For section 94 of the Act, denition reportable event, the following events are prescribed—
(a) surgery or another invasive procedure being performed on the wrong site of a patient’s body resulting
in serious harm to the patient or the death of the patient;
(b) surgery or another invasive procedure being performed on the wrong patient resulting in serious harm
to the patient or the death of the patient;
(c) the wrong surgical or other invasive procedure being performed on a patient resulting in serious harm
to the patient or the death of the patient;
(d) the unintended retention of a foreign object in a patient aer surgery or another invasive procedure
resulting in serious harm to the patient or the death of the patient;
(e) a haemolytic blood transfusion reaction caused by ABO incompatibility resulting in serious harm to
the patient receiving the blood transfusion or the death of the patient;
(f) the suspected suicide of a patient within an acute psychiatric unit or ward;
(g) an error relating to a patient’s medication resulting in serious harm to the patient or the death of the
patient;
(h) the use of physical or mechanical restraint resulting in serious harm to a patient or the death of a
patient;
(i) the use of an incorrectly positioned orogastric or nasogastric tube resulting in serious harm to a
patient or the death of a patient;
(j) the discharge or release of a patient who is a child under the age of 15 years to an unauthorised person;
(k) stillbirth;
(l) any death of a patient, or serious harm or other harm to a patient that is likely to be permanent, that—
i. is not mentioned in paragraphs (a) to (i); and
ii. was not reasonably expected to be an outcome of the health service provided to the patient.
Other factors to consider in deciding whether to do an RCA under the HHB Act include:
Condentiality
(see section
105)
RCA team members and commissioning authorities are generally prevented
from disclosing information acquired as part of an RCA. There are a number of
exceptions to this general rule, including providing RCA reports to patient safety
entities (such as the Patient Safety and Quality and statutory Quality Assurance
Committees), the Health Ombudsman and the Coroner.
Protections for
RCA reports
(see section
119)
RCA reports, chain of events documents and other documents created by (or for)
an RCA team cannot be accessed under administrative or court orders, and are
not admissible in legal proceedings other than a coronial inquest (including civil,
criminal and disciplinary proceedings).

Appendix B | 81
Protections
for RCA team
members
(see sections
104-115)
RCA team members are protected from civil liability for acts or omissions made honestly
and without negligence for an RCA and must be indemnied by the appointing authority
for the costs of defending any related proceedings.
Protections
for RCA
participants
(see sections
116-119)
RCA team members cannot be required to give evidence or produce documents relating
to an RCA in legal proceedings other than proceedings for an oence under the Act.
Sta or other persons cannot be required to give evidence in legal proceedings about:
• whether the person gave information to an RCA team
• what information the person gave to an RCA team
• a document the person gave to an RCA team that was created by the person for the
purposes of the RCA
• information the person was given, or questions the person was asked, by an RCA team.
Clinical review
The HHB Act also facilitates—but does not mandate—the use of a clinical review to identify
recommendations on ways in which the safety and quality of public sector health services can be
maintained and improved. When commissioned as a standalone review, the legislation provides
protections from clinical review reports being used in legal proceedings and provides certain protections
for those appointed to conduct the clinical review. These protections apply to clinicians appointed to
provide expert advice to Health Service Chief Executives, the Director-General and a person or entity
whose role includes maintaining and improving the safety and quality of public sector health services.
When a clinical review is undertaken to inform a HSI, this type of clinical review report is NOT privileged
and may be accessed through administrative or legal proceedings.
Similar to RCA, some of the factors to consider in deciding whether to conduct a clinical review under the
HHB Act are:
• Condentiality
Experts are generally prevented from disclosing information acquired as part of clinical review (see
section 132).
• Protections for appointed clinical review reports
Clinical review reports, except those to provide clinical advice to a health service investigator, cannot
be accessed under administrative or court orders and are not admissible in legal proceedings,
including civil, criminal and disciplinary protections (see section 138).
• Protections for appointed clinicians
Clinicians who are appointed under the HHB Act are protected from civil liability for acts or omissions
made honestly and without negligence for a clinical review (see section 280(1)(d) ).
Clinicians appointed to conduct a clinical review under the HHB Act cannot be required to produce their
report or give evidence relating to their report in legal proceedings (see section 138(3) ).
Health service investigations
The HHB Act, Part 9 also facilitates—but does not mandate—the use of health service investigation to
investigate and report on any matters relating to the management, administration or delivery of public
sector health services.
Health service investigations are not primarily a safety and quality tool and do not attract the statutory
privileges granted to RCAs and clinical reviews under the HHB Act.
If a RCA or clinical review is not appropriate, or is subsequently stopped, because the matter under
analysis is believed to involve a Blameworthy Act* (see section 94), a HSI may be the most appropriate
incident analysis tool, or if there is a mix of clinical and non-clinical issues issues, a HSI may be a flexible
option.
* A Blameworthy Act is:
(a) an intentionally unsafe act;
(b) deliberate patient abuse; or
(c) conduct that constitutes a criminal oence

82 | Best practice guide to clinical incident management Second edition - January 2023
Appendix C
A just culture approach
Source: NHS ‘A just culture guide’
Source: NHS ‘A just culture guide’ (https://www.england.nhs.uk/patient-safety/a-just-culture-guide)
(45)
A just culture approach
Supporting consistent, constructive and fair evaluation of the actions of staff involved in patient safety incidents
This guide supports a conversation between managers
about whether a staff member involved in a patient
safety incident requires specific individual support or
intervention to work safely. Action singling out an
individual is rarely appropriate -most patient safety
issues have deeper causes and require
wider action.
The actions of staff involved in an incident should not
automatically be examined using this just culture guide,
but it can be useful if the investigation of an incident
begins to suggest a concern about an individual action.
The guide highlights important principles that need to be
considered before formal management action is directed
at an individual staff member.
An important part of a just culture is being able to
explain the approach that will be taken if an incident
occurs. A just culture guide can be used by all parties
to explain how they will respond to incidents, as a
reference point for organisational HR and incident
reporting policies, and as a communication tool to
help staff, patients and families understand how the
appropriate response to a member of staff involved in
an incident can and should differ according to the
circumstances in which an error was made. As well
as protecting staff from unfair targeting, using the
guide helps protect patients by removing the
tendency to treat wider patient safety issues as
individual issues.
Please note:
• A just culture guide is not a replacement for an
investigation of a patient safety incident. Only a full
investigation can identify the underlying causes that
need to be acted on to reduce the risk of future
incidents.
• A just culture guide can be used at any point of
an investigation, but the guide may need to be
revisited as more information becomes available.
• A just culture guide does not replace HR advice
and should be used in conjunction with
organisational policy.
• The guide can only be used to take one action (or
failure to act) through the guide at a time. If multiple
actions are involved in an incident they must be
considered separately.
Start here:
Q1. Deliberate harm test
1a. Was there any intention to cause harm?
YES
Recommendation:
Follow organisational guidance for
appropriate management action. This could involve: contact
relevant regulatory bodies, suspension of staff, and referral to
police and disciplinary processes. Wider investigation is still
needed to understand how and why patients were not protected
from the actions of the individual.
END HERE
No go to this question:
Q2. Health test
2a. Are there indications of substance abuse?
YES
Recommendation: Follow organisational substance abuse at
work guidance. Wider investigation is still needed to understand
if substance abuse could have been recognised and addressed
earlier.
END HERE
2b. Are there indications of physical ill health?
YES
Recommendation: Follow organisational guidance for health
issues affecting work, which is likely to include occupational
health referral. Wider investigation is still needed to understand
if health issues could have been recognised and addressed
earlier.
END HERE
2c. Are there indications of mental ill health?
if
No to all
go to this question:
Q3. Foresight test
3a.
Are there agreed protocols/accepted practice in place that apply to the
action/omission in question?
If NO to any
Recommendation: Action singling out the individual is unlikely
to be appropriate; the patient safety incident investigation
should indicate the wider actions needed to improve safety for
future patients. These actions may include, but not be limited to,
the individual.
END HERE
3b. Were the protocols/accepted practice workable and in routine use?
3c.
Did the individual knowingly depart from these protocols?
if
Yes to all
go to next question:
Q4. Substitution test
4a.
Are there indications that other individuals from the same peer group,
with comparable experience and qualifications, would behave in the
same way in similar circumstances?
If YES to any
Recommendation: Action singling out the individual is unlikely
to be appropriate; the patient safety incident investigation
should indicate the wider actions needed to improve safety for
future patients. These actions may include, but not be limited to,
the individual.
END HERE
4b.
Was the individual missed out when relevant training was provided to
their peer group?
4c.
Did more senior members of the team fail to provide supervision that
normally should be provided?
if No to all go to this question:
Q5. Mitigating circumstances
¯
¯
5a. Were there any significant mitigating circumstances?
YES
Recommendation: Action directed at the individual may not be
appropriate; follow organisational guidance, which is likely to
include senior HR advice on what degree of mitigation applies.
The patient safety incident investigation should indicate the
wider actions needed to improve safety for future patients.
END HERE
if
No
Recommendation: Follow organisational guidance for appropriate management action. This could involve individual training, performance management,
competency assessments, changes to role or increased supervision, and may require relevant regulatory bodies to be contacted, staff suspension and
disciplinary processes. The patient safety incident investigation should indicate the wider actions needed to improve safety for future patients.
END HERE

Appendix D | 83
Appendix D
Restorative just culture framework
(29,30)
First victim/s
Who is hurt? Patients/Client/Consumer, Family, Carer
What do they need? Information about the incident
Access to the clinician/s involved
Restitution
Reassurance of prevention
Who should meet those needs? Clinician/s
Obligation and action Allow them to tell and retell their story and be willing to
participate in the process
Clinician Disclosure and Open Disclosure
Engagement in the review process
Evaluation – provide feedback
Second victim/s
Who is hurt? Clinicians / Practitioners
What do they need? Psychological rst aid, (support, compassion, healing)
Tell their story and learning - forgiveness
Reinstatement
Who should meet those needs? Organisation to have support programs/policies/ procedures –
Peer support
Obligation and action Determine the most appropriate review process Recognise needs
of rst victim/s
Express remorse
Contribute to the review and the learning
Organisation/s
Who is hurt? Directorates, Services within, Departments/Units or
Whole of organisation
What do they need? Support and learning
Information
Leverage for change
Reputational repair
Who should meet those needs? Department to have programs, policies, plans
Obligation and action Willing to participate
Oered help
Explored systemic processes – consider best practice – forward
looking review
Community
Who is hurt? Community members who witnessed or were aected by the
incident – (small regional or rural centres including indigenous)
What do they need? Regular current Information about incident and aermath via 13
HEALTH, hotlines and websites, Support and reassurance
Who should meet those needs? Department and/or Organisation
Obligation and action Development of plan to engage & communicate with community.
A willing to participate in restorative process and forgiveness

84 | Best practice guide to clinical incident management Second edition - January 2023
Appendix E
Creating a constellation diagram
The diagramming step of the analysis process is focused on recognising all system issues that may
have contributed to the incident rather than just the factors that are apparent and closer to the point
of incident occurrence. Diagramming can assist teams to better understand systemic factors and the
inter- relationships between them, better visualise these relationships, and help avoid the trap of
hindsight bias. Diagramming is one of the elements that can increase the credibility, reliability and
eectiveness of analysis in making care safer.
Many readers will be familiar with the use of Ishikawa (also called shbone)
(59)
and tree
(60)
diagrams
(Figures E.1 and E.2) to support analysis, however both these types of diagrams have limitations.
Ishikawa diagrams are helpful for brainstorming and clustering factors, but do not easily illustrate
complex relationships between factors. Tree diagrams have been perceived as too linear and their top-
down approach can be misleading in terms of relative importance of identied contributing factors.
Figure E.1: Ishikawa (shbone) diagram
Communication Training Fatigue/Scheduling
Policies/procedures
Environment/
equipment
Barriers
Critical incident
Figure E.2: Tree diagram
Action or
condition
Action or
condition
Root cause
caused by
caused by
caused by
caused by
caused by
caused by
caused by
Harmful
outcome
Action or
condition
Action or
condition
Incident
Action or
condition
Action or
condition
Action or
condition
Action or
condition
Root cause
Root cause
caused by caused by
caused by caused by
caused by caused by
In an attempt to address the advantages and limitations of these two types of diagrams, the features
of each were blended into a constellation diagram, and a new diagramming method was developed.
A literature search did not identify any references to constellation diagrams in the context used here
(diagramming and analysis methods (including statistical analysis) that emphasise the identication
of groups of elements as well as their inter-relationships—for example, the functional resonance
accident model,
(79)
concept and cognitive mapping
(80,81)
and social network analysis.
(82)
Through its suggested categories of factors and use of guiding questions, the new diagram oers
a systematic way to analyse contributing factors at the system level. In addition, the unique visual
representation of the constellation diagram encourages and facilitates the identication of inter-
connections and the sphere of influence among contributing factors, which will assist in identifying
the contributing factors with the biggest impact on patient safety.

Appendix E | 85
Improving safety and quality of care in complex adaptive healthcare systems is dependent on the
ability to see how the parts of the system influence each other so the limited resources available can
be focused with more precision to where the greatest risks are identied. The constellation diagram
oers flexibility to accomplish this, more than the Ishikawa and tree diagrams.
There are ve steps involved in developing a constellation diagram of a patient safety incident:
1. Describe the incident
2. Identify potential contributing factors
3. Dene inter-relationships between and among potential contributing factors
4. Identify the ndings
5. Conrm the ndings with the team.
The development and recording of the diagram can be done using the local resources available, such
as a hand-drawn diagram that can be scanned in an electronic format, a photograph of sticky notes,
as well as using soware like Word, Excel, Visio, Mindmap or others.
Step 1: Describe the incident
1. Briefly summarise the incident and harm/potential harm in the centre of the diagram (typically
fewer than 10 words)(Figure E.3).
Figure E.3: Describe the incident
Incident:
Outcome:
It is crucial for the team to clearly dene the starting point for the analysis. This is usually a harmful
outcome that the team wants to prevent. It is oen, but not always, the actual outcome. For example,
in the case of a near miss the potential incident may have been recognised prior to the patient being
involved. Alternatively, an incident may have occurred but was recognised and action taken prior to
harm resulting. In both of these circumstances, the analysis team would identify the starting point for
analysis as the potential harm, as no harm actually occurred.
Step 2: Identify potential contributing factors
1. Add the contributing factor categories (task, equipment, work environment, patient, care team,
organisation, etc.) to the diagram in a circle around the incident/outcome description. (Figure E.4).
2. Use the example guiding questions provided (appendix K), and other questions as appropriate, to
identify potential contributing factors.
3. Place each potential contributing factor on a sticky note and group the factors near the category
title (Figure E.5).
Figure E.4: Add contributing factors
Incident:
Outcome:
Care team
Work
environment
Organisation
Equipment
Patient
Other Task

86 | Best practice guide to clinical incident management Second edition - January 2023
Figure E.5: Dene relationships between potential contributing factors
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Incident:
Outcome:
Care team
Work
environment
Organisation
Equipment
Patient
Other
Task
Factor
Factor
When identifying potential contributing factors, focus on system-based factors to ensure that the
recommended actions are not people-focused. Keeping in mind human factors principles and systems
theory, analysis should focus on how and why certain human actions occurred, not just that they
occurred.
For instance, in the course of analysing an incident in which an incorrect medication was
administered, it was determined that the nurse was in a hurry. The fact that the nurse was in a hurry
is a factual detail of what happened, and not a contributing factor. The contributing factor/s are
those that may have caused the nurse to be in a hurry. Examples could include: too many tasks were
assigned (e.g. the nurse was assigned too many complex patients) or the patient’s medication needs
conflicted with shi change (e.g. the patient was admitted right before the shi ended and the nurse
wanted to give the patient their pain medication so that they did not have to wait until aer the shi
change). By focusing on the systems-based contributing factors, the analysis team will be able to
identify higher leverage solutions. Recommended actions should be consistent with one of the main
tenets of human factors: t the task or system to the human, not the other way around.
Step 3: Dene inter-relationships between and among potential contributing factors
1. For each potential contributing factor ask, ‘how and why did this happen?’, ‘what was this
influenced by?’ and ‘what else influenced the circumstances?’
2. Add the answers to these questions to develop relational chains:
a) some contributing factors may be directly linked with each other, within the same category, to
create a chain
b) some answers may come from dierent contributing factor categories. If so, show the linkage
by drawing lines.
3. Continue to ask ‘why’ and ‘what influenced it’ questions until no further information can be
generated.
Once the team has identied potential contributing factors using the categories of guiding questions,
the second phase of analysis begins. Asking what this was influenced by? and what else influenced
the circumstances? The team then expands the constellation diagram to include relational chains of
contributing factors as shown in gure E.6. This questioning process continues until there are no more
questions, knowledge becomes limited, or until the issues identied fall outside the scope of the
analysis. Expect that factors from dierent chains will be inter-related and may influence each other.
Appendix E | 87
Figure E.6: Dene relationships between potential contributing factors
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Incident:
Outcome:
Care team
Work
environment
Organisation
Equipment
Patient
Other
Task
Factor
Factor
Step 4: Identify the ndings
The next step in the analysis process is to identify the ndings that are central to the incident. The
team should expect to identify several ndings. There is seldom only a single reason why an incident
occurred.
Findings will be identied in three categories:
1. Factors that, if corrected, would likely have prevented the incident or mitigated the harm—these
will be the basis for developing recommended actions (note that these factors may require actions
at dierent levels of the system).
The question to be asked is ‘if this factor was eliminated or corrected, would it have likely reduced
the risk of incident recurrence and/or harm?’ While it is possible that many contributing factors will
be identied in the analysis, certain factors, if corrected, have the greatest probability to prevent
the incident altogether, or mitigate harm from the incident. It is common for these factors to be
highly relational—in other words, relationships or potential relationships between a number of
the identied factors appear to have combined to enable an incident to occur; there is a sphere
of influence amongst them. These ndings will be the basis for developing recommended actions
(note that actions may be required at dierent levels of the system).
Two options:
• would it have likely reduced the risk of the incident occurring?
• would it likely reduce the risk of similar incident from recurrence?
2. Factors that if corrected, would not have prevented the incident or mitigated the harm, but are
important for patient/sta safety or safe patient care in general. These issues should be included
in the team’s ndings and brought to the attention of the appropriate individuals for follow-up and
documented in the analysis report for future analysis and actions as appropriate.
3. Mitigating factors—factors that didn’t allow the incident to have more serious consequences and
represent solid safeguards that should be kept in place.
An example of a completed constellation diagram is illustrated in gure E.7 on the following page.
See also Appendix K for a case study, including a complicated constellation diagram.

88 | Best practice guide to clinical incident management Second edition - January 2023
Figure E.7: Completed constellation diagram
Finding
Finding
Finding
Finding
Finding
Finding
Finding
Finding
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Factor
Incident:
Outcome:
Care team
Work
environment
Organisation
Equipment
Patient
Other
Task
Factor
Factor
Step 5: Conrm the ndings with the team
1. Ensure consensus and support for the development of recommended actions.
The team should agree on the ndings before moving forward to develop recommended actions.
If there is a lack of immediate agreement, it is important to discuss and work through any
disagreements to strive to arrive at consensus before proceeding. If key individuals involved
in the incident are not participants on the analysis team, it is helpful to ask for their feedback
on the ndings of the analysis team as part of the process for verifying the ndings. This stage
of the process should also include a back-checking step—in other words, consider the impact
of correcting the identied vulnerabilities (e.g. if this factor had not been present or had been
corrected, would the incident still have occurred?).

Appendix F | 89
Appendix F
Severity assessment code (SAC) matrix
A clinical incident is dened by Australian Commission on Safety and Quality in Health Care (ACSQHC) as “an event or circumstance that resulted, or could have
resulted, in unintended and/or unnecessary harm to a patient or consumer; and/or a complaint, loss or damage.
SEVERITY ASSESSMENT
CODE (SAC)
SAC 1 SAC 2 SAC 3 SAC 4
ACTUAL CONSEQUENCE TO
PATIENT
Death or likely permanent harm which is not reasonably expected as an outcome of healthcare. Temporary harm which
is not reasonably
expected as an
outcome of healthcare.
Minimal or no harm
which is not reasonably
expected as an outcome
of healthcare.
No harm or
near miss
SOURCE: ‘Reportable
event’ in accordance with:
Section 29: Hospital and
Health Boards Regulation
2012
Reportable events
For section 94 of the Act, denition reportable
event, the following events are prescribed—
(a) surgery or another invasive procedure being
performed on the wrong site of a patient’s
body resulting in serious harm to the patient
or the death of the patient;
(b) surgery or another invasive procedure being
performed on the wrong patient resulting in
serious harm to the patient or the death of
the patient;
(c) the wrong surgical or other invasive procedure
being performed on a patient resulting in
serious harm to the patient or the death of
the patient;
(d) the unintended retention of a foreign object
in a patient aer surgery or another invasive
procedure resulting in serious harm to the
patient or the death of the patient;
(e) a haemolytic blood transfusion reaction
caused by ABO incompatibility resulting in
serious harm to the patient receiving the
blood transfusion or the death of the patient;
(f) the suspected suicide of a patient within an
acute psychiatric unit or ward;
(g) an error relating to a patient’s medication
resulting in serious harm to the patient or the
death of the patient;
(h) the use of physical or mechanical restraint
resulting in serious harm to a patient or the
death of a patient;
(i) the use of an incorrectly positioned orogastric or
nasogastric tube resulting in serious harm to a
patient or the death of a patient;
(j) the discharge or release of a patient who
is a child under the age of 15 years to an
unauthorised person;
(k) stillbirth;
(l) any death of a patient, or serious harm or other
harm to a patient that is likely to be permanent,
that—
i. is not mentioned in paragraphs (a) to (i);
and
ii. was not reasonably expected to be an
outcome of the health service provided to
the patient.
Additional information, including specic
denitions relating to some of above is available
here: Section 29: Hospital and Health Boards
Regulation 2012
Includes the following
as a result of a clinical
incident:
• Additional
investigations
performed.
• Surgical
intervention.
• Procedures involving
the wrong patient or
body part resulting
in temporary harm
Includes the following
as a result of a clinical
incident:
• First aid treatment only
required.
Includes the
following
as a result
of a clinical
incident:
• No harm
• Near miss

90 | Best practice guide to clinical incident management Second edition - January 2023
Appendix G
Guide to level/type of analysis
Level/type of analysis based on degree of harm
This table provides suggestions on what might be considered appropriate and proportionate when assessing
the level of analysis required for a clinical incident. Health services may have local policies regarding the type
of analysis for SAC2, SAC3 or SAC4 clinical incident and the reporting requirements. There may be situations
where an incident with a low SAC rating requires a comprehensive analysis based on the level of risk. When
considering if an analysis is required there a number of criteria to consider including:
• Severity of incident
• Probability of recurrence
• Complexity of the factors that influenced the incident
• Other contextual factors (preliminary assessment, frequency of occurrence, regulatory mandates, internal or
external pressures)
PATIENT SAFETY INCIDENTS
Degree
of harm
Assessment of risk Level/type of analysis based on risk of harm
NO HARM
Could have realistically resulted in
severe or death outcome
Comprehensive analysis (there is oen much to learn from
how incidents were prevented)
Incident occurring on subject where
national guidance has been issued
Comprehensive analysis (there is oen much to learn from
how incidents were prevented)
Frequently occurring
Consider multi-incident or concise analysis (combining
multiple analyses may lead to more eective solutions)
May represent signicant concern or
systemic service failure
Concise analysis or comprehensive analysis (combining
multiple analyses may lead to more eective solutions)
Attracting public concern or media
interest and not included above
Concise analysis
LOW HARM
Could have realistically resulted in
severe or death outcome
Comprehensive analysis
Incident occurring on subject where
national guidance has been issued
Concise analysis or comprehensive analysis
Frequently occurring
Consider multi-incident or concise analysis (combining
multiple analyses may lead to more eective solutions)
May represent signicant concern
or systemic service failure
Concise analysis or comprehensive analysis
(dependent on potential for future harm)
Attracting public concern or media
interest and not include above
MODERATE
Could have realistically resulted in
severe or death outcome
Comprehensive analysis
Incident occurring on subject where
national guidance has been issued
Concise analysis or comprehensive analysis
Frequently occurring
Consider concise or multi-incident analysis (combining
multiple analyses may lead to more eective solutions)
May represent signicant concern
or systemic service failure
Concise analysis or comprehensive analysis
(dependent on potential for future harm)
Attracting public concern or media
interest and not include above
SEVERE
Frequently occurring
Consider comprehensive or multiple-incident analysis
(combining multiple analyses may lead to more eective
solutions)
All other patient safety incidents,
claims or complaints with severe
outcome
Comprehensive analysis
DEATH
Homicide by or of patient in receipt
of mental health care program
approach in last 6 months
Comprehensive analysis and/or independent analysis
(set timescales)
Suicide of patient in receipt of
mental health care program
approach in last 6 months
Consider comprehensive or aggregated analysis
(combining multiple analyses may lead to more eective
solutions)
Any other potentially avoidable
deaths in healthcare or
healthcare premises
Comprehensive analysis

Appendix H | 91
Appendix H
Sample analysis team charter
Date:
From:
Subject: Incident analysis team charter
To:
1. This memorandum conrms that an analysis team will be convened to determine the contributing
factors for the patient safety incident briefly described below:
Date incident occurred ___ /___ /___ Date organisation was aware of incident ___ /___ /___
The analysis method is (tick one):
Comprehensive
Concise
Multi-Incident
Other
2. As part of the process, the team will be responsible for developing a nal report and
recommendations based on their expert analysis. All analyses are quality assurance focused
processes and the team’s products (e.g. interviews, preliminary and nal reports, etc.) are
considered condential.
This analysis is a root cause analysis and will be privileged and protected under the Hospital and
Health Boards Act 2011 ( tick one):
Yes
No
Note: If in the course of conducting the analysis it appears that the patient safety incident/s
under consideration may have been related to an intentional unsafe act or acts, the appropriate
organisational representative should be contacted to determine if an administrative analysis, or
other type of analysis process, should occur.
3. List of disciplines and/or services anticipated to be involved in the analysis:
4. List of potential internal (e.g. facility) and external experts or consultants:
5. Resources available to the team (e.g. room number, flip charts, laptop computer, etc.):
6. The team’s nal report is due on: ___ /___ /___
(Adapted from the Veterans Aairs National Centre for Patient Safety, in the Canadian Root cause analysis Guide)
(78)

92 | Best practice guide to clinical incident management Second edition - January 2023
Appendix I
Team management checklist
Team management checklist
Planning
Team members identied and conrmed
Room booked
Refreshments ordered
Preparation
Condentiality agreement
Project charter/terms of reference
Health record
Related policies and procedures
Incident sequence of events
Flip charts, sticky notes, markers
Agenda and goals, pre-reading if required
Ground rules
Follow-up
Additional meeting/s scheduled:
Report preparation delegated to: Target date: ___ /___ /___
Documents collected

Appendix J | 93
Appendix J
Investigative interview guidance (cognitive type interview)
Cognitive type interview: taking a rst-hand account of individuals’ involvement in a patient safety
incident.
An investigative interview is designed to help interviewees retrieve from memory the events
associated with a patient safety incident.
(83)
A cognitive interview is an interviewing technique based on psychological theory and research for
examining the retrieval of information from memory.
(84)
The interview style recommended for incident analysis is a modied approach of the formal cognitive
interview. It involves actively listening to someone who recalls their rst-hand account of an event they
have either witnessed, or been involved in, as soon aer it has happened as possible.
Preparation
Listening to the rst-hand accounts from those involved in an incident as soon as possible aer it has
happened will help the investigation team start to build a picture of what happened and potentially
highlight what other information will be required. The optimum time for holding an interview is
between two and 72 hours aer the incident.
(84,85)
The interviewer needs to establish who they want to interview and make arrangements to do so as
soon as possible. The identied sta should be invited to attend, told the purpose of the interview,
what to expect and what preparation they need to do. It is essential that the interviewer and the room
are prepared prior to the interview.
Inviting the member of sta to attend for an interview
Where appropriate, a written invitation to the interview can be provided and the details below
included. Where this is not practical due to the need to see sta as soon as possible aer the incident,
sta should be advised in advance and be given the following information verbally:
• The purpose of the interview and details of the incident being investigated.
• The time, place and estimated length of the interview.
• Who will be conducting the interview and their role.
• How the interview will be conducted and the rst-hand account record (e.g. the interview will be
informal, notes will be taken to inform the investigation, but these will not act as a formal witness
statement and do not need the interviewee’s signature).
• What documentary evidence will be available to them during the interview.
• The fact that they can bring a friend or colleague for support (explanations need to be given
regarding the role of this friend/colleague e.g. condentiality, their involvement etc.).
• Advice on what will happen aer the interview.
Interviewer preparation
• The interview should take place in a quiet, relaxed setting and, if possible, away from the
interviewee’s usual place of work and not at the scene of the incident.
• The room should be set out informally with refreshments available and steps taken to ensure,
where possible, no interruptions occur (e.g. mobile phones).
• Where possible, the interviewee should have the opportunity to attend the interview in work time
and arrangements may need to be made with their line manager to ensure this.
• Depending on the nature of the case or the interviewee’s personal involvement, they may nd the
process of recounting the events either upsetting or disturbing. The interviewer will need to have
information available on sta support/counselling.
• The interviewer should ensure they have all the relevant documentation available at the interview.
It is important to remember in the cognitive interview to only interview one sta member at once.

94 | Best practice guide to clinical incident management Second edition - January 2023
Conducting the interview
Introductions (where appropriate) should be made of those present in the room. Include details
on roles and an explanation of the sequence of the interview and approximate length. The incident
analysis process should be explained and an estimate given of how long it will take to complete.
It is important to reinforce that the incident analysis is not part of a disciplinary process. The
interviewer should explain that notes will be taken throughout for the purpose of informing the
investigation. It must be stressed that these notes will not act as a formal witness statement.
Guidance and support should be given by a union representative or solicitor as applicable.
The interviewee should be asked to conrm they have understood all of the above and should be
reminded that they should oer only factual information, but include everything regardless of whether
they think it is relevant or not. The interviewee should be discouraged from making o the record
comments. The interviewee should also be advised that the rst-hand account and the nal report will
be written with due anonymity to sta and the patient.
Completion of the interview
On completion, the interviewer should ensure the interviewee feels appropriately supported and that
any further support required is organised. The interviewer should reconrm what will happen with the
information gained from the interview and how this will be used in the incident analysis process.

Appendix K
Case study—comprehensive analysis: resident absconds
from a residential aged care facility
Background
The scenario for analysis is based upon a resident who absconds from a secure dementia unit within
a residential care facility. The care facility is located in a community in regional Queensland. In the
summer months, temperatures regularly reach 35 degrees celsius and in the winter, it may be as cold
as 8 degrees celsius.
In this scenerio, residents deemed to be at risk of wandering are provided with monitoring bracelets
called wanderer alarms and there are monitoring alarms at the main entrance, at the front of the care
unit (located adjacent to the front door of the building), as well as at a re exit at the back of the care
unit, which is at the rear of the building. The re exit is equipped with an alarm that sounds when
the door is opened. The monitoring bracelets are checked every couple of weeks to ensure they are
functioning properly.
Incident
At supper time, a personal care assistant noticed that a 78-year-old female resident was not in the
dining room. The personal care assistant was asked to look for her but could not nd her in the
nursing home. A code yellow was called. On notifying the police, it was learned that the resident
had been found, cold and confused, walking on a highway two kilometres away and that police were
trying to determine where she lived. The resident had been taken to a local emergency department for
assessment and treatment.
Immediate response
The nursing director and administrator were notied and took the following actions:
1. Contacted the resident’s family to advise them of the incident.
2. Instructed sta to:
a. ensure the safety of other residents by testing all door alarms and monitoring bracelets
b. secure the resident’s health record
c. quarantine the resident’s monitoring bracelet upon her return to the home
d. test the emergency exit alarms.
3. Met with the relevant sta the next morning to conduct a preliminary debrief to gather and
establish known facts, and provide emotional support, including advising about the availability of
the Employee Assistance Services (EAS).
4. Ensured completion of appropriate documentation in the health record and clinical incident report.
Appendix K | 95

96 | Best practice guide to clinical incident management Second edition - January 2023
Prepare for analysis
In the days following the incident, the nursing director and the patient safety/quality coordinator
made a preliminary assessment of the known facts related to the incident. In consultation with the
home administrator, a decision was made that a comprehensive analysis would be required. This
decision was communicated to the nursing director.
Once a decision was made to undertake a comprehensive analysis of the incident, a team was
convened who included the following:
• analysis technical expert (patient safety/quality coordinator)
• content expert (nurse manager—East Wing)
• front line worker (carer—North Wing)
• person from another discipline (so what person) (receptionist).
Analysis process—what happened?
Prior to the rst meeting with the analysis team, the patient safety/quality coordinator undertook
some preliminary information gathering for the analysis team:
• Interviewed all sta directly involved (e.g. all sta working the day and evening shi that day,
including carers, medical sta, nursing sta, etc.).
• Interviewed others who may have helpful information (e.g. the resident’s family, other family
visitors).
• Reviewed the resident’s health record for relevant clinical information.
• Reviewed organisational policies and procedures related to monitoring of residents with cognitive
decits.
• Contacted other local residential care facilities for copies of policies and procedures related
to monitoring of residents with cognitive decits and reviewed the current state and national
guidelines.
At the rst meeting with the analysis team, the team:
1. Analysed information gathered by the patient safety/quality coordinator:
a) Information from the incident report:
i. 78-year-old female resident found two kilometres from the care facility by local police.
Resident was distressed and confused.
ii. Temperature 10 degrees celsius.
iii. Resident dressed in light clothing and slippers.
iv. Resident transported to local emergency department for assessment and treatment.
v. Police received call from the care facility indicating that resident was missing - police
advised that resident has been transported to hospital.
vi. Resident assessed in emergency department, treated with warm blankets and IV fluids and
observed overnight.
vii. Resident returned to the care facility the following morning aer breakfast.
b) Policies and procedures related to monitoring of residents considered a risk for absconding.
c) Results of a literature search and environmental scan for current best practices related to
management of residents who are at risk for absconding.
2. Visited the secure unit in the care facility and walked around pertinent areas, including the
resident’s room, the dining room and the lounge, checking for the location of exits and alarms and
conducted a safe simulation of the incident.
3. Examined monitoring devices available for use and reviewed manufacturer’s instructions.
4. Created a detailed sequence of events of the incident (Table K.1).

Appendix K | 97
Table K.1: Detailed sequence of events for abscond incident
Date/time Information item Comment/
source
Four months
prior to incident
• 78-year-old female resident admitted to the secure
dementia unit of the home.
• Medical history: type 2 diabetes, dementia.
• Admission medications: Metformin 500mg three times
daily, Donepezil 5mg daily, multiple vitamins daily.
• Initial nursing assessment: impaired cognition,
poor decision-making skills, mild confusion, walks
independently with a cane.
• Assessed as a risk for absconding and a monitoring
bracelet was placed on her right wrist.
Health record;
sta interviews
Six weeks prior
to incident
Resident has become increasingly confused and agitated.
Assessed by physician who ordered Risperidone 0.25mg at
bed time.
Nursing progress
notes
Four weeks prior
to incident
Resident found outside the home in the early evening.
Resident was in the sta parking lot at the back of the
building and was found by a sta member coming in for
the evening shi. Sta on duty did not recall hearing any
alarms sounds. The resident’s bracelet was tested and
found to be working.
Nursing progress
notes, sta
interview
Two weeks prior
to incident
Resident very confused and attempting to leave unit,
redirected numerous times by sta. Doctor contacted, order
received to increase Risperidone to 0.25mg twice daily.
Nursing progress
notes
Day of incident
1145hr
Resident told nurse who gave noon medications that she
was going home. Sta planned for resident to eat lunch in
the dining room and then nap in her room per her usual
routine. She was last observed eating lunch.
Sta interview
1305hr Back door alarm sounded, reset by sta without checking
as one sta member had just le the desk on lunch break
and usual practice was to exit through back door to gain
easy access to the parking lot.
Sta interview
1600hr Carer went to check on resident to get her ready for dinner
but did not nd her in her room, assumed she was already
in the lounge watching TV.
Sta interview
1730hr Carers noticed that resident was not in the dining room.
Discussed with other sta who went to check her room.
Sta interview
1740hr Carers unable to locate resident. Checked other care units
and walked around perimeter of building but could not
locate her.
Health record,
sta interviews
1755hr Carer reported to charge nurse that resident is missing. A
code yellow alert was called, with a comprehensive search
initiated of the entire facility.
Health record,
sta interviews

98 | Best practice guide to clinical incident management Second edition - January 2023
Date/time Information item Comment/
source
1840hr Sta unable to locate resident on the grounds. Resident’s
family contacted. Evening sta are arriving so three of
the day shi sta use their personal vehicles and begin
searching the surrounding area. Call made to local police.
Police advise that an elderly woman was found two
kilometres from the home at approximately 1800hrs and
that she has been transported to hospital for assessment
as she was distressed (dressed only in light clothing and
slippers, temperature 10 degree Celsius) and appeared
confused.
Health record,
sta interviews
1845hr Resident’s family contacted to advise that resident has
been found and is being cared for at the local emergency
department.
Health record,
sta interviews
1850hr CNC at the care facility contacts the local emergency
department for report on resident condition.
Resident has had IV fluids initiated and has been given
warm blankets.
Health record,
sta interviews
1900hr CNC contacts nursing director to provide report of
situations.
Health record,
sta interviews
Day aer
incident
0930hr
Resident returned to residential aged care facility from
hospital.
Health record
1030hr The resident’s monitoring bracelet is removed and tested.
Found not to be working. It was later determined that the
resident had been provided with a 90 day device, rather
than a 12-month device as intended.
Health record
Analysis process: how and why it happened
At the second analysis team meeting, the team used information provided in the sequence of events
and their understanding of the incident from the simulation to create a constellation diagram (Figure
K.1). The following steps are required to create a constellation diagram:
1. Describe the incident:
− outcome: resident found distressed and dehydrated two kilometres from the care facility
− incident: resident absconded.
2. Identify potential contributing factors using contributing factor categories and guiding questions
3. Dene relationships between contributing factors
4. Identify ndings
5. Validate the ndings with the team.

Appendix K | 99
Figure K.1: Constellation diagram of abscond incident
Care team
Work
environment
Organisation
Equipment
Patient
Other
Task
Action or
condition
No
standardised
process for
“mock” codes
Process
changes not
implemented
aer previous
absconding
issue
Close call
charted but
not formally
reported
investigation
Sta
unfamiliar
with Code
Yellow
procedures
Code Yellow
not fully
implemented
when resident
rst identied
as missing
Communication
lacking betweek
team members
when resident
rst identied as
missing
Cognitively
impaired
elopement
risk
Fire alarm
not “heard”
or responded
to
Fire alarm
sounds
frequently
sta are
desensitised
Electronic
bracelet
failed to
alarm
Code Yellow not
called when
resident not in
room
Lack of clarity
around when to
call a Code Yellow
Assumptions
made re:
resident’s
whereabouts
Lack of standard
expectations re:
resident status
checks
No internal
process to
ensure device
testing and
accompanying
documentation
Routine use of
an emergency
exit to access
the sta
parking lot
Two types of
bracelets
stocked
Similar
appearance
of device
3 month
device
used
instead of
12 months
Monitoring
bracelet
was
expired
Electronic
bracelet not
tested daily per
instructions
prodvided with
device
Caregivers
initially
worked
independently
to try to nd
resident

100 | Best practice guide to clinical incident management Second edition - January 2023
Statements of ndings
The analysis team identied the following ndings:
• Task:
− Lack of standard expectations regarding resident status checks decreased the likelihood that
the resident absconding would be detected in a timely way.
• Equipment:
− Two types of monitoring bracelets with similar appearance stocked in the care facility increased
the likelihood that the incorrect device would be selected and applied.
− No standardised internal process to ensure testing of monitoring bracelets with accompanying
documentation decreased the likelihood that the bracelet would be identied as non-
functioning prior to an absconding incident.
• Work environment:
− Routine use of an emergency exit to access the sta parking lot decreased the likelihood that
the alarm function would be eective as sta became desensitised to frequent alarms.
• Patient:
− The resident’s cognitive impairment decreased the likelihood that she would be aware of the
risk of leaving the facility.
• Care team:
− Communication lacking between team members when resident rst identied as missing,
combined with lack of familiarity with Code Yellow procedures, decreased the likelihood that a
Code Yellow would be initiated immediately.
• Organisation:
− Lack of a formal process to report and investigate close calls decreased the likelihood that a
Code Yellow would be initiated immediately, would be followed-up to identify process changes
to prevent future occurrences.
− Lack of a standardised process for regular mock codes to provide ongoing training and assess
sta understanding of processes decreased the likelihood that sta would be familiar with
Code Yellow procedures.
• Other
− No other factors identied.
Analysis process: What can be done to reduce the risk of recurrence and make care safer?
The analysis team proposed the following recommended actions:
• Task (T):
− T1: Establish routine procedures for conrming and documenting whereabouts of residents
with wandering dementia.
• Equipment (E):
− E1: Develop a standardised process for daily checks, with documentation, of monitoring
bracelets.
− E2: Standardise devices used to monitor residents at risk of absconding to either the 90 day or
12-month model.
• Work environment (W):
− W1: Implement magnetic card access technology to enable sta use of the emergency exit door,
elimination frequent nuisance alarms.
• Organisation (O):
− O1: Work with frontline sta to develop and apply criteria for reportable incidents.
− O2: Develop a protocol for analysing high risk near miss incidents to ensure that learning is
applied to prevent recurrence (e.g. by using the concise incident method).
− O3: Ensure sta members are familiar with the Code Yellow protocol through a scheduled in-
service and ongoing inclusion in orientation sessions.
− O4: Ensure sta members are procient in the use of the Code Yellow and other emergency
protocols through quarterly unscheduled mock code exercises.

Appendix K | 101
Prioritise actions
Recommendation
Risk
severity
assessment)
Hierarchy of
eectiveness
(high,
medium, low
leverage)
Predictors
of success
(alignment,
existing
mechanisms,
quick wins)
System level
targeted
(micro,
meso, macro,
mega)
Note if evidence is
available and what
type
Conrm
validity,
feasibility
Order of priority (or
timeframe)
T1: Establish routine procedures for conrming and documenting
whereabouts of residents with wandering dementia
High Medium Medium Micro No Medium Within 30 days
E1: Develop a standardised process for daily check, with
documentation, of monitoring bracelets.
High Medium High Micro Yes, other unit is
doing daily checks
successfully
High Within 30 days
E2: Standardise devices used to monitor residents at risk of
absconding to either the 90 day or 12-month model.
Medium High Low Meso Yes, Global Patient
Safety Alerts
Medium Within 6 months
W1: Implement magnetic card access technology to enable sta
use of the emergency exit door, eliminating frequent nuisance
alarms.
Medium High Medium Meso No Medium Within 12 months
O1: Work with frontline sta to develop and apply criteria for
reportable incidents.
High Low High Meso No Medium Within 6 months
O2: Develop a protocol for analysing high risk/
Near miss incidents to ensure that learning is applied to prevent
reoccurrence (e.g. by using the concise incident analysis
method).
High Low High Macro No High Within 6 months
O3: Ensure sta are familiar with the Code Yellow protocol
through a scheduled in-service and ongoing inclusion in
orientation sessions.
High Low High Micro No High Within 30 days
O4: Ensure sta are procient in the use of the Code Yellow
protocol through quarterly unscheduled mock Code Yellow
exercises.
High Low High Meso Yes, simulation
research paper XYZ
High First mock code
to be held within
3 months

102 | Best practice guide to clinical incident management Second edition - January 2023
Follow through
Evaluation implementation.
The Director of Care analysed the status of implementation of recommended actions one year aer the incident analysis was completed.
Recommendation (category)
Sources
and ID#
Date
entered
Progress status Timeframe (end date) Target area Risk
level
Individual responsible
E1: Standardise daily device checks with
documentation.
IA # ID Sept. 13 Implemented as
presented Oct. 1
Oct. 1 All residents High Director of Care
E2: Standardise devices to either the 90
day or 12- month model.
IA # ID Sept. 13 Under
consideration
All residents High Director of Purchasing
W1: Magnetic card access technology for
emergency exists.
IA # ID Sept. 13 Nothing done All emergency Medium Director of Purchasing
O1: Development and application of
criteria for incident reporting.
IA # ID Sept. 13 Partially
implemented
New reporting form
implemented in June
All sta High Director of Care
O2: Protocol for analysis of high risk near
miss incidents.
IA # ID Sept. 13 Partially
implemented
Two near miss events
analysed (May and July)
All sta High Director of Care
O3.1: Code Yellow in service for all sta. IA # ID Sept. 13 Implemented as
presented
Completed Oct. 15 and 20 All sta in home High Director of Care
O3.2: Code Yellow inclusion in orientation. IA # ID Sept. 13 Implemented as
presented
January orientation session All new sta High Director of Human Resources
O4: Quarterly unscheduled mock Code
Yellow exercises.
IA # ID Sept. 13 Steps towards
implementation
One mock code held Feb. 20 All new sta in home
Patient
High Patient Safety Leader

Appendix L | 103
Appendix L
Incident analysis guiding questions
A set of guiding questions is provided below to guide the identication of contributing factors,
hazards and mitigating factors during the how and why did it happen stage of incident analysis.
They are intended to assist with checking the availability and strength of safeguards at all levels in
the organisation. The questions assist to guide the analysis towards the identication of system
vulnerabilities that aligned in such a way that allowed for the incident to take place. Teams are
encouraged to note, analyse and report the system barriers that worked well (mitigating factors) and
which should be reinforced so they will continue to prevent future harm.
The questions are grouped around categories of factors designed to focus the analysis on the
interaction between humans and the system, and in this way help identify system-level contributing
factors at various levels in the organisation. The categories were developed by researching and
adapting categories used in analysis throughout the world
(49,50,51,52)
and rened through pilot testing
and consultation with a human factor specialist.
The way the list is used is a matter of personal preference. Some may choose to use the questions
below to guide information gathering and interviews, while others may prefer to use them to cross-
reference the information already collected. The goal of this exercise is to go through the questions
to nd if the safeguards were in place and functioning. For each category, consider what other factors
may have contributed to the incident and include them in the analysis.
• The guiding questions are provided as examples—this is not an exhaustive list.
• The guiding questions are not intended to be used as the interview questions, but as prompts
for analysis.
• For every guiding question, ask how it impacts the incident.
• If the answer to a guiding question suggests that the safeguard was not in place or did not
work, probe further with additional questions (e.g. why is this the case? If so, how did this/
these contribute to/impact the incident?).
Task (care/work process):
• Were there previous or predicted failures for this task or process?
• Were specialised skills required to perform the task?
• Was a xed process or sequence of steps required (e.g. order sets, checklists)? Did it exist and was
it followed?
• Was a protocol available, was it up-to-date and was it followed in this case?
• Were there constraints or pressures (e.g. time, resources) when performing the task?
• Was the information required to make care decisions available and up-to-date (e.g. test results,
documentation, patient identication)?
• Was there a risk assessment/audit/quality control program in place for the task/process?
• Other?
Equipment (including information and communication systems):
• Were the displays and controls understandable?
• Did the equipment automatically detect and display problems?
• Was this display functional?
• Were the warning labels, reference guide and safety mechanisms functional and readily visible/
accessible?
• Were the maintenance and upgrades up-to-date?
• Was the equipment standardised?
• Would the users describe this equipment as easy to use?
• Were the communication systems (phone, pager, soware, hardware etc.) available and
operational?
• Other?

104 | Best practice guide to clinical incident management Second edition - January 2023
Work environment:
• Did noise levels interfere with the alarms?
• Was the lighting adequate for the task?
• Was the work area adequate for the task/s being performed (e.g. space, layout, location and
accessibility of resources)?
• Other?
Patient/s characteristics:
• Did the patient/s have information to assist in avoiding the incident? If not, what would have
supported the patient in assisting their care team?
• Did factors like age, sex, medications, allergies, diagnosis or any other medical conditions
contribute to the incident? How did they contribute?
• Did any social or cultural factors contribute to the incident? What factors? In which way?
• Was language a barrier?
• Other?
Care team:
Caregiver/s:
• Was the education, experience, training and skill level appropriate?
• Was fatigue, stressors, health or other factors an issue?
• Was the workload appropriate?
• Was appropriate and timely help or supervision available?
• Other?
Supporting team (all involved in care process):
• Was there a clear understanding of roles and responsibilities?
• Was the quality and quantity of communication (verbal and/or written) between team members
appropriate (clear, accurate, free of jargon, relevant, complete and timely)?
• Were there regular team briengs/debriengs about important care issues?
• Was team morale good? Do team members support each other?
• Were the communication channels available and appropriate to support the needs of the team
(e.g. email, pager and phone)?
• Other?
Organisation:
Policies and priorities:
• Were the relevant policies and procedures available, known, accessible and did they meet the
needs of users?
• Were there workarounds to the documented policy/procedure?
• Was there a mechanism in place to identify and resolve gaps between policy and practice?
• Were the strategic priorities of the organisation clear to all?
• Other?
Culture:
• Was everyone (patients, clinicians, other sta) comfortable to speak up about safety concerns?
• Was there visible support from leadership and board for safe patient care?
• Was communication between sta and management supportive of day-to-day safe patient care?
• Were incidents considered system failures with people not blamed?
• Other?

Appendix L | 105
Capacity (resources):
• Did scheduling influence the stang level, or cause stress, fatigue?
• Was there sucient capacity in the system to perform eectively (e.g. access to resources)?
• Were targets and/or incentives appropriate?
• Other?
Other—consider:
• Were there any local conditions or circumstances that may have influenced the incident and/or an
outcome?
• Were there any sector specic conditions or circumstances that may have influenced the incident
and/or outcome?
• Other?

106 | Best practice guide to clinical incident management Second edition - January 2023
Appendix M
Three human factors methods that can be used in incident analysis
Various human factors methods can be employed in the analysis process to help answer the question
how did it happen? They range in complexity, time and resources, and expertise (in human factors)
needed. All three methods (described below) assist in examining the human-system interaction
in detail. With cognitive walkthrough—perhaps the easiest and most cost-eective method to
employ —a participant is asked to think out loud as they simulate the tasks that were involved in the
incident. In a heuristic evaluation, an audit is carried out of the various parts of the system (such as
equipment, paper forms, computer systems etc.) that were used in the tasks that were part of the
incident. The audit is used to determine if human factors design principles were violated, and as such,
may be identied as possible contributing factors in the incident. Heuristic evaluation requires an
understanding of human factors principles as they apply to dierent systems (e.g. computer systems).
Finally, usability testing can be used, in which human-system interaction with equipment, paperwork,
or processes are observed (similar to a simulation). Participants are asked to carry out a set of tasks
in a simulated environment given the scenario in the incident. Some level of human factors training
is needed in order to plan and execute usability tests, and to interpret the results. However, the
information is extremely helpful and detailed because, if done correctly, the usability test examines
how the human-system interaction occurs in the real world.
Cognitive walkthrough
As noted above, this is perhaps the quickest to conduct and takes the least amount of time, resources
and human factors expertise to complete, as compared to the two other methods discussed here.
Cognitive walkthrough can be used to help identify contributing factors in the analysis phase, or it is
used to help discover the details of the cognitive and physical activities that took place (or may take
place, in the case of evaluating a recommended action).
To carry out a cognitive walkthrough, recruit participants who are either representative of the person/s
involved in the incident (e.g. pharmacist or nurse) or the actual workers involved, to simulate the set of
tasks surrounding the incident. Ask the participant to think out loud as they simulate, or walk through
each step of that task. The key is that they verbalise what they are thinking as they are doing it.
Throughout the simulation, it is helpful to ask prompting questions such as ‘what were you looking to
do at this point?’, ‘what did you have to gure out?’, ‘where did you nd the information you needed?’,
‘what did you have to think about next?’, ‘what made you think you needed to do that?’, ‘how obvious
was it to you?’ or ‘how condent were you that you did it correctly?’.
The success of a cognitive walkthrough is heavily dependent on:
• The participant feeling comfortable to express their thoughts without fear.
• The proper identication of the task or activities that participants will simulate (if the task is too
narrowly dened, it will limit the amount of information you can nd).
• The facilitator of the cognitive walkthrough keeping their opinions to themself and not leading the
participant (the facilitator should only tell the participant what task to perform, but not how they
should perform the task, nor how they should have performed the task).
If possible, recruit between one and six people to participate in the walkthrough. It is best to have
four to six participants because it will capture a wider cross-section of the human-system interaction.
However, one participant is better than none and even one person will provide extremely rich
information for the incident analysis.
At the end of the cognitive walkthrough, the person conducting the activity will have a more detailed
understanding of the cognitive and physical activities that led to the incident and what aspects of the
system may have failed to support these activities, and therefore may have been contributing factors.

Appendix M | 107
Alternatively, if the cognitive walkthrough was conducted to evaluate proposed recommended action,
the walkthrough will provide some insight into their eectiveness. It may also help determine if the
recommended action has created some unintended and undesirable consequences, such as:
• Does it take additional unnecessary mental eort?
• Does it make the task overly complex or tedious?
• Does it create confusion or uncertainty?
• Does it create risk for other kinds of errors?
Depending on the response to these questions, it may be necessary to modify or select an alternate
recommended action to pursue (and possibly evaluate again using any of the three human factors
methods described in this appendix).
Heuristic evaluation
This method requires some knowledge of human factors design principles and how to apply them
to specic systems (e.g. computer systems). It may take approximately the same amount of time to
conduct as the cognitive walkthrough, though possibly longer depending on complexity, and does
not require participants or other special arrangements. This method can be useful in the analysis
phase to help identify contributing factors, or to help evaluate recommended actions before they are
implemented.
In a heuristic evaluation, an audit of the system is performed to determine if human factors design
principles are violated. The principles cover a wide range of issues related to whether the design of the
system ts the task or human. The audit can identify where human-system interaction is negatively
influenced.
The results of a heuristic evaluation can provide very detailed information about contributing factors
and how they can be changed to improve the risk for errors. Also, the method can be used to help
develop and design the recommended action.
Usability testing
Among the three methods described here, usability testing likely takes the most time and resources.
It also requires some expertise in human factors to plan, execute and analyse the results. However
simple usability tests can be performed that are not as time and resource consuming and can yield
very helpful information about contributory factors, or about whether a recommended action is
eective.
In a usability test, participants are recruited to carry out a specic task (or set of tasks). The test can
be carried out in a simulated setting, or in some cases the actual work area. Then information related
to how the task (or set of tasks) was executed is gathered, such as time on task, number (and nature)
of steps, or errors. This allows for observation of how the human-system interaction plays out, and
where diculties are encountered (contributing factors). A formal usability test may require anywhere
from 20 to several hundred participants and take weeks, if not months, of planning. However, for
the purpose of gathering information for an incident analysis, a less formal approach can be taken,
and fewer participants recruited, because the aim is to gain a qualitative understanding of possible
contributing factors. Four to six participants would be desirable, but even involving only one or two
participants may yield helpful qualitative information for the incident analysis.
Similar to other methods described, usability testing can be used for both identifying contributing
factors as well as for evaluating the eectiveness of recommended actions.

108 | Best practice guide to clinical incident management Second edition - January 2023
Example of using human factors to guide an incident analysis.
When examining an incident in which a nurse incorrectly sets up a medical device, it is important
to identify the contributing factors. An action, such as ‘the nurse pushed the wrong button’ is
not a contributing factor; it is a factual description of what happened. The goal in the analysis is
to determine how and why this happened. To approach this question using human factors, it is
necessary to examine the equipment’s user interface and look for design features that may have
influenced this action. For instance, as part of a heuristic evaluation, questions you could ask
include:
• Was the button close to the one they intended to push?
• Was it labelled in a manner that led them to believe that pushing that button was the correct
action?
• Were the instructions that were displayed on the screen unclear as to what button they needed
to push next?
• Was the button label inconsistent with the terminology used in the displayed instructions?
• Was the button grouped closely with other buttons that are typically used in the task the nurse
was performing (leading them to believe that it was to be used in this task)?
• Was the button’s appearance similar to (and possibly confusable with) other buttons?
• Were there other confusing features on the interface that may have caused a misunderstanding
or confusion?
You could also look at materials that were involved in setting up the device. For instance, if an
order form was used, you would examine its ease of use. Not only it’s readability and legibility, but
also how it related to the task of setting up the device. For instance:
• Does the nurse refer to the order form during device set up?
• What information does the nurse use to help with the set up?
• Is the information provided in a logical order that matches what they need to do with the
device?
• Is the terminology used on the order form consistent with what’s used on the device?
• Is there any information that may be confusing?
• Does the organisation of the information on the order form match the flow of the task?
Next, you would explore the nature of the task and how that may have influenced the human-
system interaction. For instance, time pressure, performing multiple tasks at once, complexity of
the steps, and so forth. Also, the environment, work area layout, organisation context, team and
patient factors also may influence how work is carried out and therefore may be the source of
contributing factors.
The guiding questions in Appendix L provide a starting point for examining the factors that may
have played a role in the incident.
A cognitive walkthrough to observe nurses setting up the device will also provide information
on aspects of the process that may be confusing, or where information is not readily available,
leading to interruptions in the process that may also lead to errors.

Appendix N
Developing a statement of ndings template and examples
Developing a statement of finding - TEMPLATE
‘The contributing factor/s, within the context of the incident, increased/decreased the likelihood
that this outcome would occur’.
Primary Outcome
Immediate
What happened?
Intermediate
Why did it happen?
Root cause
If we change, may
prevent future harm
Statement of finding
led to,
Intermediate
contributed to,
Immediate
increased the likelihood of,
Problem statement
Appendix N | 109

110 | Best practice guide to clinical incident management Second edition - January 2023
Developing a statement No 2 table
Developing a statement of finding – EXAMPLE 1
‘The contributing factor/s, within the context of the incident, increased/decreased the likelihood
that this outcome would occur’.
Primary Outcome
a patient overdose of prescribed medication
Immediate
What happened?
patient received twice the prescribed quantity of dopamine
Intermediate
Why did it happen?
incorrect programming of the IV pump
Root cause
If we change, may
prevent future harm
Statement of finding
The lack of nursing induction training in IV pump operation
led to,
Intermediate
the incorrect programming of the IV pump which
contributed to,
Immediate
to the patient receiving twice the prescribed quantity of dopamine
which
increased the likelihood of,
Problem statement a patient overdose of prescribed medication.
Developing a statement No 3 table
Developing a statement of finding - EXAMPLE 2
‘The contributing factor/s, within the context of the incident, increased/decreased the likelihood
that this outcome would occur’.
Primary Outcome
skin lesion being removed from the wrong limb
Immediate
What happened?
a bypass of routine final checking
Intermediate
Why did it happen?
a culture of non-compliance
Root cause
If we change, may
prevent future harm
Statement of finding
the absence of practise compliance monitoring
led to,
Intermediate
a culture of non-compliance which
contributed to,
Immediate
a bypass of routine final checking that
increased the likelihood of,
Problem statement a skin lesion being removed from the wrong limb.
Source: ACHS Improvement Academy - https://www.achs.org.au/improvement-academy
Source: ACHS Improvement Academy - https://www.achs.org.au/improvement-academy

Appendix O | 111
Appendix O
Case study—concise analysis: medication incident
Background
This scenario takes place within a community that is serviced by a hospital and busy home care
service. The hospital emails new and updated home care referrals to a generic email address. The
referral form provides demographic patient information, diagnosis, a list of discharge medications
and doctor’s orders for home care. During business hours Monday to Friday, a home care coordinator
analyses the emailed document and accesses the Home Care Central Record for any existing patients.
The coordinator then analyses the information in the documents and schedules the applicable home
care visits. Aer business hours and on weekends, home care nursing sta periodically check the
emails and sorts them by ongoing patients or new patients. Referrals updating the status of ongoing
patients are given directly to one of the nurses responsible for the geographic area of the community.
Pharmacists and technicians dispense medications from the pharmacies in the community.
Technicians are responsible for processing prescriptions in the computer and preparing and labelling
medications as well as inventory management functions. Pharmacists are responsible for analysing
the patient medication prole and completing the nal check of the medications before they are
dispensed for pick-up or home delivery.
Some attending doctors at the community hospital email prescriptions to patients’ pharmacies so that
patients and families can easily pick up any medications needed on the way home.
Incident
The incident involves a 78 year old male homecare patient requiring a leg ulcer dressing change
every ve to seven days. The patient is obese and has a history of angina, high blood pressure and
deep vein thrombosis. He has limited mobility and was in hospital for eight days with a diagnosis
of community acquired pneumonia. The patient was discharged on a Saturday with a referral sent
through the home care email account to advise of his return home. His list of medications were noted
on the form as: Nifedipine 10mg tds (calcium channel blocker), Atenolol 50mg bd (beta blocker),
Coumadin 2 mg daily (anticoagulant), Aspirin 100mg daily (antiplatelet), doxcycline 100mg daily x 6
days (antibiotic), nitrospray prn and DuoDERM dressing to leg ulcer weekly. Additional background
information: patient was weak and slightly short of breath at discharge.
Analysis process – what happened?
Based on the incident report, an analysis of the home care record, hospital chart and referral form,
the facilitator responsible for conducting this concise analysis started to dra a sequence of events of
the incident (Table O.1). The interviews conducted with the patient, pharmacist and registered nurses
(RNs), together with an examination of the drugs involved in the incident, helped conrm and expand
the sequence of events.

112 | Best practice guide to clinical incident management Second edition - January 2023
Table O.1: What happened: medication incident—nal sequence of events
Date/time Information item Comment/source
History Patient receiving weekly home care visit by RN for leg
ulcer dressing change every ve to seven days for
approximately six weeks. Occasionally forgetful about
caring for dressing and short-term memory mildly
impaired, however able to manage own medications.
Friday, 14 days
prior to event.
Five days prior
to event
RN makes home visit to change patient’s leg dressing.
She notes that he is feverish and short of breath with
congested cough. RN contacts patient’s family general
practitioner and transfer to hospital is arranged. Patient
is admitted with community acquired pneumonia.
Home care record
Five days prior
to event
Patient is discharged from hospital and returns to
apartment. INR testing during hospital stay resulted in
Warfarin dose being reduced to 2mg daily. Physician
referral lists medications Nifedipine 10 mg tds (calcium
channel blocker), Atenonol 50mg bd (beta blocker),
Coumadin 2mg daily (anticoagulant), Aspirin 100mg
daily (antiplatelet), Doxycycline 100mg daily x 6 days
(antibiotic), Nitrospray prn and Duo DERM dressing
to leg ulcer weekly and request to resume dressing
change schedule as well as request for assistance
with weekly bath. Referral received by fax on Saturday.
RN responsible for that area of the community on the
weekend does not know the patient however she
analysed referral and home care record. Minimal changes
noted so booked for RN visit for dressing change in ve
days (Thursday) and home care aide booked to make
home visit for assistance with bath in six days (Friday).
She leaves a voice mail for the regularly scheduled RN
in the area to advise her of the patient’s return home
however, that RN is o work for several days before
receiving the message. RN has signicant backlog of
messages and workload so does not take any action with
this information.
Hospital chart and
referral form
RN interview
(regularly
scheduled in the
area)
Five days prior
to event
Neighbour picked up patient to bring him home. She
agreed to pick up the new prescription when getting
groceries later that day. The pharmacist at the pharmacy
gave a patient information sheet with a new prescription.
The neighbour provided this to the patient.
Patient exhausted on the day he returned home from
hospital. Grateful to neighbour for ride home and getting
his prescription as well as groceries. He does recall the
neighbour saying to read the information sheets but
couldn’t nd his glasses and was too tired. He noted the
two new pills and daily dose directions. He added them
to his medication regimen until the one pill bottle was
empty.
Patient interview

Date/time Information item Comment/source
Five days prior
to event criteria
for reportable
event
At the drug store:
• pharmacy technician processes lling the
prescription in computer
• pharmacist notes the change in Warfarin/Coumadin
dose from 3mg daily to 2mg daily so ensures
that new bottle of tablets provided for ease of
self administration. All medications are lled for
dispensing to ensure that patient has sucient
supply for upcoming month
• pharmacist attempts to explain dosing information
to neighbour. Highlights the dose change on the
patient information sheets as well as the potential of
increased anticoagulant eect with the combination
of Doxycycline and Warfarin
Pharmacist
analysis
Four days prior
to event
Patient continues to feel tired and is not eating or
drinking very much. Spends much of the day resting is in
bed or watching TV.
Patient interview
Two days prior
to event
Patient feels weaker and more concerned about colour
of urine and more blood in stool. Doesn’t want to bother
neighbour so decides to wait for nurse visit in two days
for dressing change.
Patient interview
One day prior to
the event
Patient slept in bed most of day and doesn’t recall many
other details.
Patient interview
Day of event at
0900hrs
Patient was found in bathroom by RN on arrival at
0900hrs for dressing change. Moderate amount of bright
red blood in toilet and floor. Ambulance called and
transferred to ED.
RN interview
Day of event
1400hrs
RN called ED and spoke with charge nurse. Patient’s
INR 5.8. Upon analysis of medication bottles it was
determined that patient was unintentionally taking 5mg
of Warfarin daily as he did not know that Coumadin was
the same medication as Warfarin so took previously
ordered dose of 3mg and newly prescribed dose of 2mg
as well.
RN interview
2 days aer
event
Patient remains in hospital but is recovering and should
be ready to return home soon.
Hospital chart
Analysis process – how and why it happened
The facilitator created a constellation diagram (Figure O.1) to visualise and better understand the
factors that contributed to the incident and their interconnections. The factors were conrmed by
consultation with those engaged in the incident and operational and/or medical leaders. This step
was very helpful in summarising the ndings and developing recommended actions.
Appendix O | 113

114 | Best practice guide to clinical incident management Second edition - January 2023
Figure O.1: Constellation diagram of medication incident
Care team
Work
environment
Organisation
Equipment
Patient
Other
Task
Weekend RN and
regularly assigned RN
did not have time to call
or visit Patient soon
aer discharge
Pharmacy/ Pharmacist
did not conrm Patient’s
understanding of new
start/stop anticoagulant
doses
No MedRec/ communi-
cation process for
patients returning home
from hospital with new
prescriptions
Deterioration in
Patient’s
medical
condition (and
ability for self
care) not
detected upon
discharge
home
Discharge
MedRec not
routinely
completed for
patients leaving
hospital
Patient not
identied by
hospital, GP
or home care as
requiring nursing
assessment
Pharmacy/
Pharmacist did
not communicate
anticoagulant
dose change to
home care
services
Incident:
Anticoagulant
overdose
Outcome:
Patient experienced
rectal bleeding and
hospitalisation
Hospital care
team did not
complete
discharge
MedRec and/or
provide an
updated BPMH
to pharmacy,
patient or home
care service
Patient and
home care
service not
aware of
anticoagulant
dose change
Patient did
not know
that
Coumadin
and Warfarin
were the
same drug
Patient unable
to interact
directly with the
pharmacist or
analyse drug
information
Patient recently
unable to obtain
and manage
anticoagulants
independently
Patient
unintentionally
self administered
Coumadin
and Warfarin
There are no
guidelines/protoco
ls or risk
assessment tools
to guide the
identication of at
risk patients
leaving hospital
No system
approach to
MedRecand/orsingl
ae source of truth
for up to date Best
Possible
Medication History
Reduced physical & cognitive
abilities related to age and
medical condition

Statements of ndings
• Task:
− No key ndings.
• Equipment:
− No key ndings.
• Work Environment:
− The lack of a standardised home care risk assessment tool or protocol increased the likelihood
that patients discharged from hospital back to the community would not be accurately triaged
to ensure appropriate and timely home care services are provided.
• Patient:
− The deterioration in the patient’s physical and cognitive abilities increased the likelihood of a
medication error in his self-medication management.
• Care team and organisation:
− The lack of a formalised, system-wide and communicated Discharge Medication Reconciliation
Process (including an updated best possible medication history) decreased the likelihood
that the patient would receive the appropriate and timely support required for safe medication
management.
− No other factors identied.
Analysis process—what can be done to reduce the risk of recurrence and make care safer:
• Work environment (W):
− W1: Establish a standardised home care risk assessment tool for screening patients that are
transitioning back to the community from hospital. Consider the feasibility and eectiveness
of the regularly assigned home care nurse beginning the screening process with a call from the
acute care nurse planning for the patient discharge then completing the assessment with a
telephone or in-person patient assessment.
• Care team and organisation (CO):
− CO1: Develop, implement and evaluate a system-wide Discharge Medication Reconciliation
Process. Consider using a pilot test approach initially to determine a successful strategy for
spread.
Appendix O | 115

116 | Best practice guide to clinical incident management Second edition - January 2023
Prioritise actions
Recommendation
Risk
severity
assessment)
Hierarchy of
eectiveness
(high,
medium, low
leverage)
Predictors
of success
(alignment,
existing
mechanisms,
quick wins)
System level
targeted
(micro, meso,
macro, mega)
Note if evidence is
available and what type
Conrm
validity,
feasibility
Order of
priority (or
timeframe)
W1: Develop, implement and evaluate a standardised
home care risk assessment tool for screening patients that
are transitioning back to the community from hospital.
Medium Medium Medium Micro, meso,
macro
Expert opinion, related risk
assessment tools validated
in peer reviewed literature
Medium Within 3
months
CO1: Develop, implement and evaluate a Discharge
Medication Reconciliation Process Pilot.
Medium Medium High Micro, meso,
macro, mega
Yes, peer analysed
research and expert
opinion
Medium Within 6
months
Follow through
An evaluation was completed by the Quality Improvement (QI) Director one year aer the incident analysis was completed:
Recommendation (category)
Sources
and ID#
Date
entered
Progress status Timeframe (end date) Target area Risk level Individual
responsible
W1.1: Develop standardised home care risk assessment tool. 1A # 1A June 13 Implemented as
presented
Developed and
approved Aug. 13
Home Care Medium Home Care
Executive Director
W1.2: Implement standardised home care risk assessment
tool.
1A # 1B June 13 Implemented as
presented
Implemented Oct. 13 All current and
new sta
Medium Home Care
Executive Director
W1.3: Evaluate standardise home care risk assessment tools. d1A # 1C June 13 Steps toward
implementation
In progress Chart audit —
home care
Medium QI Director
CO1.1: Develop MedRec Pilot. 1A # 1D June 13 Implemented as
presented
Developed and
approved Oct. 13
Home care Medium QI Director
CO1.2: Implement MedRec Pilot. 1A # 1E June 13 Implemented as
presented
Implemented Nov. 13 Home care Medium Medical Director for
Home Care
CO1.3: Evaluate MedRec Pilot. 1A # 1F June 13 Steps toward
implementation
In progress Home care Medium Medical Director for
Home Care
CO1.4: Share MedRec evaluation with organisational decision
makers for decision regarding spread to system wide
implementation.
1A # 1G June 13 Not
implemented
Home care Medium Medical Director for
Home Care

Appendix P | 117
Appendix P
Lessons learned
Lessons can be derived from any activity. They are a product of operations, exercises, training,
experiments and day-to-day sta work. During the course of our activities most of us will recognise
ways of doing things more easily or eciently that can be passed on to our colleagues and successors
to help them avoid problems and do even better than we did before. The challenge facing any
organisation is to build a culture within which we all feel comfortable and motivated to share our
knowledge in a productive way.
(86)
In the course of learning lessons, we exploit both explicit and tacit knowledge.
Learning from explicit and tacit knowledge:
• Explicit knowledge is knowledge that has been or can be documented. This type of knowledge
can lead to a lesson learned by the use of a lesson learned process, lesson learned
information sharing tools, such as databases and training courses.
• Tacit knowledge is knowledge that has not or cannot be documented but is still extremely
valuable. This type of knowledge is stored in our heads and can lead to a lesson learned when
we interact with others by discussion and sharing experience within a community, perhaps
facilitated by formal working groups, conferences or other events.
In any learning organisation, regardless of whether you are learning from explicit or tacit knowledge,
you will follow the same three basic stages of learning.
Three basic steps to learning:
1. Identication: collect learning from experiences.
2. Action: take action to change existing ways of doing things based on the learning.
3. Institutionalisation: communicate the change so that relevant parts of the organisation can
benet from the learning
.(86)
Activities used to promote learning from experience can vary across organisations.
Common ways to learn from experience:
• Lessons learned process: to gather, sta, action and communicate lessons to ensure learning
from experience is converted into actual improvement via a formal process.
• Lessons learned information sharing: to make use of the databases, spreadsheets, websites,
reports or other media to store and communicate lessons.
• Lessons learned community: to bring together subject matter experts at working groups,
training courses, conferences and other events to share experience and learning.
Lessons learned summary:
• Lessons learned describes activities relating to learning from experience to achieve improvements.
In context, this means reduced patient harm, operational risk, increased eciency and improve
operational eectiveness.
• Lessons can be derived from any activity—daily events, exercises, training, etc.
• Learning, in any organisation, involves three generic stages: identication, action and
institutionalisation.
Role of the Patient Safety Unit/Clinical Governance Unit
• Support the lesson learned process—gather, analyse, sta, action and communicate lessons to
ensure learning from the experience is converted into actual improvement.
Prioritise actions
Recommendation
Risk
severity
assessment)
Hierarchy of
eectiveness
(high,
medium, low
leverage)
Predictors
of success
(alignment,
existing
mechanisms,
quick wins)
System level
targeted
(micro, meso,
macro, mega)
Note if evidence is
available and what type
Conrm
validity,
feasibility
Order of
priority (or
timeframe)
W1: Develop, implement and evaluate a standardised
home care risk assessment tool for screening patients that
are transitioning back to the community from hospital.
Medium Medium Medium Micro, meso,
macro
Expert opinion, related risk
assessment tools validated
in peer reviewed literature
Medium Within 3
months
CO1: Develop, implement and evaluate a Discharge
Medication Reconciliation Process Pilot.
Medium Medium High Micro, meso,
macro, mega
Yes, peer analysed
research and expert
opinion
Medium Within 6
months
Follow through
An evaluation was completed by the Quality Improvement (QI) Director one year aer the incident analysis was completed:
Recommendation (category)
Sources
and ID#
Date
entered
Progress status Timeframe (end date) Target area Risk level Individual
responsible
W1.1: Develop standardised home care risk assessment tool. 1A # 1A June 13 Implemented as
presented
Developed and
approved Aug. 13
Home Care Medium Home Care
Executive Director
W1.2: Implement standardised home care risk assessment
tool.
1A # 1B June 13 Implemented as
presented
Implemented Oct. 13 All current and
new sta
Medium Home Care
Executive Director
W1.3: Evaluate standardise home care risk assessment tools. d1A # 1C June 13 Steps toward
implementation
In progress Chart audit —
home care
Medium QI Director
CO1.1: Develop MedRec Pilot. 1A # 1D June 13 Implemented as
presented
Developed and
approved Oct. 13
Home care Medium QI Director
CO1.2: Implement MedRec Pilot. 1A # 1E June 13 Implemented as
presented
Implemented Nov. 13 Home care Medium Medical Director for
Home Care
CO1.3: Evaluate MedRec Pilot. 1A # 1F June 13 Steps toward
implementation
In progress Home care Medium Medical Director for
Home Care
CO1.4: Share MedRec evaluation with organisational decision
makers for decision regarding spread to system wide
implementation.
1A # 1G June 13 Not
implemented
Home care Medium Medical Director for
Home Care

118 | Best practice guide to clinical incident management Second edition - January 2023
• Support lessons learned information sharing—share lessons both within and outside of the
organisation via (but not limited to) databases, websites, reports, newsletters, etc.
• Analyse and disseminate inside the organisation pertinent lessons learned information shared by
others
• Support the lessons learned community—attend and organise relevant lessons learned sharing
events (lessons learned conferences, forums, working groups, etc.).
• Support lessons learned capability—set up or improve the organisation’s lessons learned
capability.
Learning culture—actions you can take:
• In your local clinical governance/patient safety or board meeting, analyse the
recommendations from incident investigations carried out in your facility over the previous
twelve months.
• Discuss with your colleagues whether the recommendations were implemented and have been
sustained over time. Check whether new colleagues and junior sta, who have joined your
team aer the investigation had been completed, are aware of the incident and understand
why the recommendations made in the report are important.
• Repeat this self reflection task on a bi-monthly basis—it can help avoid organisational amnesia
of previous lessons learned.
• Challenge and conrm what data is available to the board as evidence that recommendations
have been implemented and sustained over time. Remember that you are looking for evidence
that recommendations are being monitored and sustained—verbal assurances are insucient.
• Consider how you integrate improvement actions and analysis in the daily work of the
organisation to ensure that better results are sustained and spread throughout the
organisation.

Glossary | 119
Glossary
Except where referenced, the following list of terms are taken are taken from the Conceptual Framework
for the International Classication for Patient Safety.
(87)
This harmonised language allows clinicians,
organisations and countries to classify like incidents similarly, enabling the patient safety community to
share and compare information about incidents in order to learn and improve patient care.
(87)
The Queensland Department of Health, Patient Safety and Quality, Clinical Excellence Queensland,
encourages the use of these preferred terms for consistency and clarity, but also recognises that
organisations may have reason to continue to use other terminology.
Term Description
Adverse event An unexpected and undesired incident directly associated with the care or
services provided to the patient.
Best practices Clinical, scientic or professional practices that are recognised by a majority of
professionals in a particular eld. These practices are typically evidence based
and consensus-driven.
Clinician
disclosure
The informal process where the treating clinician informs the patient /family/
carer of the occurrence of an adverse event and an apology for the occurrence of
the event.
(11)
Contributing
factor
A circumstance, action or influence which is thought to have played a part in
the origin or development of an incident or to increase the risk of an incident.
Degree of harm The severity and duration of harm, and the treatment implications, that results
from an incident.
Findings 1. Factors that, if corrected, would likely have prevented the incident or
mitigated the harm—these will be the basis for developing recommended
actions (note: that these factors may require actions at dierent levels of the
system).
2. Factors that if corrected, would not have prevented the incident or mitigated
the harm, but are important for patient/sta safety or safe patient care in
general.
3. Mitigating factors—factors that prevented the incident from resulting in
more serious consequences and provide solid safeguards that should be
kept in place.
Forcing
functions
Something that prevents the behaviour from continuing until the problem has
been corrected.
Formal open
disclosure
Open disclosure is the discussion with a patient about a clinical incident
resulting in harm which was not reasonably expected as an outcome of the
health care provided.
(11)
Harm Any physical or psychological injury or damage to the health of a person,
including both temporary and permanent injury.
Human Error A term usually used to delineate one category of potential causes for
unsatisfactory activities or outcomes… Studies in a variety of elds show that
the label human error is prejudicial and unspecic.
Human factors Study of the interrelationships between humans, the tools, equipment and
methods they use, and the environments in which they live and work.

120 | Best practice guide to clinical incident management Second edition - January 2023
Term Description
Incident
(clinical)
An event or circumstance that resulted, or could have resulted in unintended
and/or unnecessary harm to a patient or consumer; or a complaint, loss or
damage. An incident may also be a near miss.
(16)
Just culture A just culture is a culture is an environment that seeks to balance the need to
learn from mistakes and the need to take disciplinary action.
Near miss An event or situation that could have resulted in an accident, injury or illness, but
did not, either by chance or through timely intervention.
Patient A person who is a recipient of healthcare. Synonyms for patient include
‘consumer’ and ‘client’.
Patient safety Actions undertaken by individuals and organisations to protect health care
recipients from being harmed by the eects of health care services.
Patient safety
entity
An entity, specied within the Hospital and Health Boards Act 2011, whose
responsibilities include the planning, implementation, management and
evaluation of patient safety initiatives and programs for a health service.
Preventable
event
An event that could have been anticipated and prepared for, but that occurs
because of an error or other system failure.
Quality
improvement
Quality improvement is the framework used to systematically improve care.
Quality improvements seeks to standardize processes and structure to reduce
variation, achieve predictable results, and improve outcomes for patients,
healthcare systems, and organisations.
(67)
Restorative
just culture
Restorative just culture aims to repair trust and relationships damaged aer
an incident. It allows all parties to discuss how they have been aected, and
collaboratively decide what should be done to repair harm.
(30)
Risk
assessment
An assessment that examines a process in detail, including sequencing of
events; assesses actual and potential risk, failure, or points of vulnerability; and,
through a logical process, prioritizes areas for improvement based on the actual
or potential patient care impact (criticality).
Root cause
analysis (RCA)
A systematic process of investigating a critical incident or an adverse outcome
to determine the multiple, underlying contributing factors. The analysis focuses
on identifying the latent conditions that underlie variation in performance and,
if applicable, developing recommendations for improvements to decrease the
likelihood of a similar incident in the future.
Safety 1 Safety 1 takes accidents as the focus point and tries to prevent harm occurring.
(8)
Safety 11 Safety 11 emphasis is on ensuring that as much as possible goes right, expanding
beyond the area of incident prevention to promoting a real safety management
approach over a simple risk assessment.
(8)

Glossary | 121
Term Description
Safety culture Organizations with eective safety cultures share a constant commitment to
safety as a top-level priority, which permeates the entire organization. Noted
components include:
(1) acknowledgment of the high-risk, error-prone nature of an organization’s
activities
(2) a blame-free environment where individuals are able to report errors or close
calls without punishment
(3) an expectation of collaboration across ranks to seek solutions to
vulnerabilities, and
(4) a willingness on the part of the organization to direct resources to address
safety concerns.
Severity
Assessment
Code (SAC)
Queensland Health SAC applies four SAC categories to capture the severity and
duration of harm that results from an incident.
• SAC1 death or likely permanent harm which is not reasonably expected as an
outcome of healthcare
• SAC2 temporary harm which is not reasonably expected as an outcome of
healthcare
• SAC3 minimal harm which is not reasonably expected as an outcome of
healthcare
• SAC4 no harm or near miss
Surveillance Routine collection and review of data to examine the extent of a disease, to follow
trends, and to detect changes in disease occurrence.
Systems
analysis
An analysis of the resources (personnel, facilities, equipment, materials, funds,
and other elements), organization, administration, procedures, and policies
needed to carry out a given task. The analysis typically addresses alternatives in
each category, and their relative eciency and eectiveness.
System failure The common categories [of systems failure] include failures of design (process
design, task design, and equipment design) and failures of organization and
environment (presence of psychological precursors such as conditions of the
workplace, schedules, etc.; inadequate team building; and training failures).
System
improvement
The result or outcome of the culture, processes, and structures that are directed
toward the prevention of system failure and improvement of safety and quality.
Underlying
cause
The systems or process cause that allow for the proximate cause of an event to
occur. Underlying causes may involve special-cause variation, common-cause
variation, or both.

122 | Best practice guide to clinical incident management Second edition - January 2023
References
1. Trew M, Nettleton S, Flemons W. Harm to Healing: Partnering with patients who have been
harmed. Edmonton, AB: Canadian Patient Safety Institute. 2011.
2. Reason J. Human error: models and management. BMJ. 2000 Mar;320:768-770.
3. Institute for Healthcare Improvement. National Patient Safety Foundation. RCA2: Improving Root
Cause Analyses and Actions to Prevent Harm. Boston, MA: 2015. [Accessed 5 Aug 2020]. Available
from: http://www.ihi.org/resources/Pages/Tools/RCA2-Improving-Root-Cause-Analyses-and-
Actions-to-Prevent-Harm.aspx
4. Australian Commission on Safety and Quality in Health Care. National Safety and Quality
Health Service Standards. 2nd ed. – version 2. Patient safety denition [Internet]. Sydney (AU):
ACSQHC 2021. [Accessed 5 Aug 2020]. Available from: https://www.safetyandquality.gov.au/
our-work/indicators-measurement-and-reporting/patient-safety-culture#defining-patient-safety-
culture%C2%A0
5. Australian Commission on Safety and Quality in Health Care. Australian Health Service Safety and
Quality Accreditation scheme [Internet]. Sydney (AU): ACSHQHC 2021. [Accessed 5 Aug 2020].
Available from: https://www.safetyandquality.gov.au/standards/national-safety-and-quality-
health-service-nsqhs-standards/assessment-nsqhs-standards/australian-health-service-safety-
and-quality-accreditation-scheme
6. Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health
Service Standards. 2nd ed. – version 2. Sydney (AU): ACSQHC; 2021.
7. Vincent C. Patient Safety. 2nd ed. Hoboken (USA): Wiley-Blackwell; 2010.
8. Hollnagel E. Safety-I and Safety-II: the Past and Future of Safety Management. Farnham (UK):
Ashgate Publishing Limited; 2014.
9. Waterson P, Jenkins DP, Salmon PM, Underwood P. ‘Remixing Rasmussen’: the evolution of
AcciMaps within systemic accident analysis. Appl. Ergon. 2017;59(Pt B):483-503.
10. Stanton NA, Salmon PM, Walker GH, Stanton M. Models and methods for collision analysis: A
comparison study based on Uber collision with a pedestrian. Safety Science. 2019;120:117-128.
11. Queensland Health. Open Disclosure Guide. Open and honest communication with patients,
families and carers. [Internet] Brisbane (AU): Queensland Health Intranet; 2020 [cited 3 Jun 2020].
Available at: https://qheps.health.qld.gov.au/psu/od/open-disclosure-information
12. Queensland Health. What is Open Disclosure? [Internet]. Brisbane (AU): Queensland Health
Intranet [Accessed 15 Feb 2022]. Available from: https://qheps.health.qld.gov.au/psu/od/open-
disclosure
13. Safer Care Victoria. Consumer representative involvement in adverse event reviews: A guide for
health services 2019. [cited 12 Dec 2021]. Available from: https://www.safercare.vic.gov.au/
support-and-training/partnering-with-consumers/health-services/involve-consumers-in-incident-
reviews
14. Oce of the Health Ombudsman. For providers [Internet]. QLD (AU): Oce of the Health
Ombudsman. [Accessed 23 Nov 2021]. Available from: https://www.oho.qld.gov.au/for-providers
15. Zimmerman TM, Amori G. Including patients in root cause and system failure analysis: Legal and
psychological implications. Journal of Healthcare Risk Management. 2007;27(2):27-34.
16. Australian Commission on Safety and Quality in Health Care. Incident Management Guide.
Sydney: ACSQHC; 2021. [cited 22 Feb 2022]. Available from: https://www.safetyandquality.gov.
au/publications-and-resources/resource-library/incident-management-guide
17. The Joint Commission. 2017. Sentinel Event Alert. Issue 57, March 1, 2017. Revised: June 18,
2021. [Accessed 13 Sep 2021]. Available from: https://www.jointcommission.org/-/media/tjc/
documents/resources/patient-safety-topics/sentinel-event/sea-57-safety-culture-and-leadership-
final2.pdf

References | 123
18. Conway J, Federico F, Stewart K, Campbell MJ. Respectful Management of Serious Clinical Adverse
Events. 2nd ed. IHI Innovation Series White Paper. Cambridge (USA): Institute for Healthcare
Improvement; 2011.
19. Weick KE, Sutclie KM. Managing the Unexpected: Resilient performance in an age of uncertainty.
San Francisco (USA): Jossey Bass; 2007.
20. Models of HRO. High Reliability Organising.
21. Wu AW, editor. The Value of Close Calls in Improving Patient Safety: Learning how to avoid and
mitigate patient harm. Oak Brook (USA): Joint Commission Resources; 2010.
22. Vincent C. Patient Safety. 2nd ed. Hoboken NJ: Wiley-Blackwell; 2010. P.119-140.
23. World Health Organization. Patient safety incident reporting and learning systems: technical
report and guidance. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
24. Dekker S. Just Culture Restoring Trust and Accountability in Your Organization. 3rd ed. Boca Raton
(USA): CRC Press; 2017.
25. Reason J. Managing the Risks of Organizational Accidents. Ashgate Publishing; 1997.
26. Marx D. Patient Safety and the “Just Culture”. A primer for health care executives. Medical Event
Reporting System – Transfusion Medicine (MERS-TM). New York, NY: Columbia University; 2001
Apr.
27. Boysen P. Just Culture: A Foundation for Balanced Accountability and Patient Safety. The Ochsner
Journal. 2013 Fall;13(3):400-406.
28. Kirkup B. NHS Improvement’s Just Culture Guide: good intentions failed by flawed design. Journal
of the Royal Society of Medicine. 2019;112(12):495-497.
29. Turner K, Stapelberg N, Sveticic J, & Dekker S. (2020). Inconvenient truths in suicide prevention:
Why a Restorative Just Culture should be implemented alongside a Zero Suicide Framework. Aust
N Z J Psychiatry, 54(6), 571-581. Available from: https://doi.org/10.1177/0004867420918659
30. Dekker S. Restorative Just Culture Checklist. 2018. [Accessed 15 Jun 2021]. Available via: https://
www.safetydifferently.com/wp-content/uploads/2018/12/RestorativeJustCultureChecklist-1.pdf
31. Hollnagel E, Wears RL, Braithwaite J. From Safety-I to Safety-II: A White Paper. The Resilient Health
Care Net: Published simultaneously by the University of Southern Denmark, University of Florida,
USA, and Macquarie University, Australia.
32. Institute of Medicine. Crossing the Quality Chasm: A new health system for the 21st century.
Washington, DC: The National Academy Press; 2001. Appendix B.
33. Sargut G, McGrath RG. Learning to live with complexity. Harvard Business Review. 2011
Sep;89(9):66-76.
34. Hollnagel E, Braithwaite J, Wears R, editors. Resilient Health Care. Burlington (USA): Ashgate;
2015.
35. Edmondson AC. Strategies for learning from failure. Harvard Business Review. 2011 Apr;89(4):48-
55.
36. Dekker S. The Field Guide to Human Error Investigations. Aldershot (UK): Ashgate Publishing;
2002.
37. International Ergonomics Association. What is ergonomics? [Internet]. Geneva (SW): International
Ergonomics Association [Accessed 24 Jun 2021]. Available from: https://iea.cc/what-is-
ergonomics/
38. Human Factors: Technical Series on Safer Primary Care. Geneva: World Health Organization; 2016.
Licence: CC BY-NC-SA 3.0 IGO
39. Braithwaite J, Churruca K, Ellis L, Long J, Clay-Williams R, Damen N, Herkes J, Pomare C, Ludlow K.
Complexity Science in Healthcare – Aspirations, Approaches, Applications and Accomplishments:
A White Paper. Australian Institute of Health Innovation, Macquarie University (AU); 2017.
40. Sheps S, Cardi K, Pelletier R, Robson R. (2015). Revealing resilience through critical incident
narratives: a way to move from Safety-I to Safety-II. In Wears R, Hollnagel E, & Braithwaite J. (Eds.),
Resilient Health Care, Volume 2 The Resilience of Everyday Clinical Work. Farnham (UK): Ashgate
Publishing, Ltd. 2015: 189-206.
41. Java A. Sphere of Influence. Social Media Research Blog. Baltimore, MD: TypePad. 2008 Aug.

124 | Best practice guide to clinical incident management Second edition - January 2023
42. Nelson EC, Godfrey MM, Batalden PB, Berry SA, Bothe AE Jr, McKinley KE, et al. Clinical
Microsystems, part 1. The building blocks of health systems. Joint Commission Journal on Quality
and Patient Safety. 2008 Jul;34(7):367-378.
43. Merriam-Webster, Inc. Context. Dictionary and Thesaurus. Springeld (USA): Merriam-Webster;
2012.
44. Zecevic A, Salmoni AW, Lewko JH, Vandervoort AA. Seniors Falls Investigative Methodology (SFIM):
A systems approach to the study of falls in seniors. Canadian Journal on Aging. 2007; 26(3):281-
290.
45. National Health Service England. A Just Culture Guide [Internet]. UK: NHS Improvement 2018
[Accessed 8 Jun 2020]. Available from: https://www.england.nhs.uk/patient-safety/a-just-culture-
guide/
46. Veterans Health Administration. VHA National Patient Safety Improvement Handbook.
Washington, DC: Department of Veteran Aairs, Veterans Health Administration; 2011 Mar.
47. Lucchaiari C, Pravettoni G. Cognitive balanced model: a conceptual scheme of diagnostic decision
making. Journal of Evaluation in Clinical Practice. 2012 Feb;18(1):82-88.
48. Dekker S. Just Culture: Balancing safety and accountability. Burlington (USA): Ashgate Publishing;
2007.
49. Canadian Medical Protective Association. Learning from Clinical incidents: Fostering a just culture
of safety in Canadian hospitals and health care institutions. Ottawa (CA): Canadian Medical
Protective Association; 2009.
50. Joint Commission Resources. Failure Mode and Eects Analysis in Health Care: Proactive risk
reduction. 3rd ed. Oakbrook Terrace (USA): Joint Commission Resources; 2010.
51. Womack JP, Jones DT. Lean Thinking: Banish waste and create wealth in your corporation. 2nd ed.
New York (USA): Free Press; 2003.
52. Lee C, Neily J, Mills PD, Hirschler KH. How to make the most of actions and outcome measures.
NCPS TIPS. 2004 Jul/Aug;4(3).
53. Ishikawa K. Introduction to Quality Control. Productivity Press; 1990.
54. Leebov W, Ersoz CJ. The Health Care Manager’s Guide to Continuous Quality Improvement. Lincoln
(USA): Authors Choice Press; 2003.
55. World Health Organisation. High 5s project. World Health Organisation; 2011 Sept
56. Zecevic AA, Salmoni AW, Lewko JH, Vandervoort AA, Speechly M. Utilisation of the seniors falls
investigation methodology to identify system-wide causes of falls in community-dwelling seniors.
The Gerontologist. 2009 Oct;49(5):685-696.
57. Canadian Patient Safety Institute. Global Patient Safety Alerts. Edmonton (CA): Canadian Patient
Safety Institute; 2011.
58. Institute for Safe Medication Practices (ISMP) Canada. Failure Mode and Eects Analysis (FMEA).
Toronto (CA): Institute for Safe Medication Practices Canada; 2009.
59. Doran GT. There’s a S.M.A.R.T. way to write management objectives. Management Review.
1981;71(11, AMA Forum);35-36.
60. Brighton B, Bhandari M, Tornetta P 3rd, Felson DT. Hierarchy of evidence: From case reports to
randomised controlled trials. Clinical Orthopaedics and Related Research. 2003 Aug;(413):19-24.
61. Health Quality and Safety Commission New Zealand. Recognising human factors and strategies
for preventing errors [Internet]. NZ. Health Quality and Safety Commission New Zealand; [2015].
[Accessed 7 May 2021]. Available from: Error-prevention-strategies-Mar-2015.pdf (hqsc.govt.nz)
62. Event Analysis Roundtable. Vancouver (CA): Canadian Patient Safety Institute; 2010.
63. White JL. Root Cause Analysis: A review of the relevant literature. Edmonton (CA): Canadian
Patient Safety Institute; 2009.
64. Queensland Health, Risk Analysis Matrix. [Accessed 7 May 2021]. Available from: https://qheps.
health.qld.gov.au/csd/business/risk-and-audit-services/risk-services/toolkit
65. Deming WE. 3rd Ed. The New Economics For Industry, Government, Education. MIT Press,
Cambridge, MA. 2018.
66. Liker JK, Meier D. The Toyota Way Field Book. New York (USA): McGraw Hill; 2006.

References | 125
67. Langley GJ, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A
practical approach to enhancing organisational performance. 2nd ed. San Francisco (USA):
Jossey-Bass; 2009.
68. Boston Consulting Group. DICE: How to beat the odds in program execution. 2012.
69. Department of Evaluation and Research Services. Dierentiation of Research, Quality
Improvement and Program Evaluation. 2010.
70. Benneyan JC. Number between g-type statistical quality control charts for monitoring adverse
events. Health Care Management Science. 2001;4:305-318.
71. Kotter JP, Schlesinger LA. Choosing strategies for change. Harvard Business Review;
1979;57(2):106-114.
72. Provost LP, Murray SK. The Health Care Data Guide: Learning from data for improvement. San
Francisco (USA): Jossey-Bass; 2011.
73. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in
healthcare processes. BMJ Quality & Safety. 2011;20(1):46-51.
74. Solberg LI, Mosser G, McDonald S. The three faces of performance measurement: Improvement,
accountability and research. Joint Commission Journal on Quality Improvement. 1997;23(3):135-
147.
75. Safer Healthcare Now! Improvement Guides: Getting Started Kit. Edmonton (CA): Safer
Healthcare Now!: 2011. Updated: [Accessed 30 Sep 2022]. Available from: https://www.
patientsafetyinstitute.ca/en/toolsResources/ImprovementFramework/Documents/
Improvement%20Frameworks%20GSK%20EN.PDF
76. Cartwright S. Double-loop Learning: a Concept and Process for Leadership Educators. Journal of
Leadership Education. 2002;1(1).
77. Pham JC, Kim GR, Natterman JP, Cover RM, Goeschel CA, Wu AW, et al. ReCASTing the RCA:
An improved model for performing root cause analysis. American Journal of Medical Quality.
2010;25(3):186-191.
78. Bagian JP, King BJ, Mills PD, McKnight SD. Improving RCA performance: the Cornerstone Award
and the power of positive reinforcement. BMJ Quality & Safety. 2011 Nov;20(11):974-982.
79. Hollnagel E. Cognitive Reliability and Error Analysis Method (CREAM). Oxford (UK): Elsevier
Science; 1998.
80. Trochim W, Kane M. Concept mapping: an introduction to structured conceptualisation in health
care. International Journal for Quality in Health Care. 2005 Jun;17(3):187-191.
81. Snider C, Kirst M, Abubakar S, Ahmad F, Nathens AB. Community-based participatory research:
development of an emergency department-based youth violence intervention using concept
mapping. Academic Emergency Medicine. 2010;17(8):977-885.
82. Anderson C, Talsma A. Characterising the structure of operating room stang using social
network analysis. Nursing Research. 2011 Nov-Dev;60(6):378-385.
83. National Patient Safety Agency, National Reporting and Learning Service. Root Cause Analysis
Investigation Tools: Investigative interview guidance (cognitive type interview). London (UK):
NHS; 2008.
84. Geiselman E, Fisher R. Investigative Interviewing—Psychology and Practice. Chichester (UK):
1999.
85. Vincent C, Adams S, Chapman J, Hewett D, Prior S, Strange P, et al. A Protocol for the Investigation
and Analysis of Clinical Incidents. RSM; 1999.
86. NATO, Joint Analysis & Lessons Learned Centre; 2011.
87. World Health Organization. World Alliance for Patient Safety. More than Words: Conceptual
Framework for the International Classication for Patient Safety. Geneva: World Health
Organisation; 2009 Jan. Report No: WHO/IER/PSP/2010.2. 2009

Best practice guide to clinical incident management
Second Edition – January 2023
