
1 | P a g e
THE NHSN STANDARDIZED
INFECTION RATIO (SIR)
A Guide to the SIR
(Based on 2015 National Baseline)
Updated March 2024
The Standardized Infection Ratio (SIR) is the primary summary measure used
by the National Healthcare Safety Network (NHSN) to track healthcare-
associated infections (HAIs). As NHSN grows, both in its user-base and
surveillance capability, the SIR continues to evolve. Highlighting the SIR and
changes resulting from an updated baseline, this document is intended to
serve both as guidance for those who are new to this metric as well as a useful
reference for more experienced infection prevention professionals.

2 | P a g e
CORRECTIONS AND UPDATES AS OF March 2024
Recent changes to this document are listed here:
• Page 3: Corrections were made to the Table of Contents
• Page 11: The picture and description of the NHSN analysis reports treeview were updated to
reflect the current version of the NHSN application
• Page 33: Updated language referring to the CMS Care Compare website
• Page 34: Updated reference to the CMS Hospital Inpatient Prospective Payment System (IPPS)

3 | P a g e
Table of Contents
Overview of the Standardized Infection Ratio (SIR) ______________________________ 4
Calculating the Number of Predicted Infections ___________________________________________ 5
Example: Logistic Regression Model (SSI) _________________________________________________ 5
Example: Negative Binomial Regression Model ____________________________________________ 8
Finding and Interpreting SIRs in NHSN _______________________________________ 11
How do I Interpret the SIRs? __________________________________________________________ 12
SIR Guide Supplement: Risk Adjustment Factors Included in the SIR, 2015 Baseline ___ 14
Introduction to the SIR Guide Supplement ______________________________________________ 14
CLABSI – Central Line-Associated Bloodstream Infection ___________________________________ 15
MBI-LCBI – Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infection ________________ 20
CAUTI – Catheter-Associated Urinary Tract Infection ______________________________________ 23
VAE – Ventilator-Associated Events ____________________________________________________ 27
a) Total VAE in Long-Term Acute Care Hospitals (LTACHs)_______________________________ 27
b) Infection-related Ventilator-Associated Complication (IVAC) Plus in LTACHs ______________ 27
c) Total VAE in Acute Care Hospitals (ACHs) __________________________________________ 28
d) Infection-related Ventilator-Associated Complication (IVAC) Plus in ACHs ________________ 31
SSI – Surgical Site Infections __________________________________________________________ 33
MRSA Bacteremia Laboratory-Identified Events __________________________________________ 37
Clostridioides difficile (CDI) Laboratory-Identified Events ___________________________________ 39
Using an Intercept-Only Model to Calculate the Number of Predicted Events ________ 42
Additional Resources _____________________________________________________ 43
ADDENDUM TO THE NHSN GUIDE TO THE SIR _________________________________ 45
Hospital Outpatient Department (HOPD) Procedure/SSI SIR Model ___________________________ 46
Outpatient Procedure Component Surgical Site Infections (OPC SSI) __________________________ 48

4 | P a g e
Overview of the Standardized Infection Ratio (SIR)
What is the SIR?
The standardized infection ratio (SIR) is a summary measure used to track HAIs at a national, state, or local level
over time. The SIR adjusts for various facility and/or patient-level factors that contribute to HAI risk within each
facility. The method of calculating an SIR is similar to the method used to calculate the Standardized Mortality
Ratio (SMR), a summary statistic widely used in public health to analyze mortality data. In HAI data analysis, the
SIR compares the actual number of HAIs reported to the number that would be predicted, given the standard
population (i.e., NHSN baseline), adjusting for several risk factors that have been found to be significantly
associated with differences in infection incidence. In other words, an SIR greater than 1.0 indicates that more
HAIs were observed than predicted; conversely, an SIR less than 1.0 indicates that fewer HAIs were observed
than predicted. SIRs are currently calculated in NHSN for the following HAI types: central line-associated
bloodstream infections (CLABSI), mucosal barrier injury laboratory-confirmed bloodstream infections (MBI-LCBI),
catheter-associated urinary tract infections (CAUTI), surgical site infections (SSI), Clostridioides difficile infections
(CDI), methicillin-resistant Staphylococcus aureus bloodstream infections (MRSA), and ventilator-associated
events (VAE).
Why not rates?
In the past, NHSN has published annual HAI rates for device-associated infections. These rates, or pooled means,
were calculated using aggregate data reported to NHSN. The total number of infections was divided by the
applicable number of device days for that time period. However, a problem with strictly using pooled mean
rates is that they cannot reflect differences in risk between populations, and therefore lose comparability over
time and across entities. For example, calculating rates from two facilities serving entirely different patient
populations can lead to an unfair comparison. One solution to this problem is the stratification of pooled means,
as was done with location-stratified CLABSI and CAUTI pooled means. However, this method only allows for
comparison of rates within strata and does not lend itself to calculating an overall performance metric for a
facility.
Instead, the SIR allows users to summarize data by more than a single stratum (e.g., location or procedure
category), adjusting for differences in the incidence of infection among the strata. For example, NHSN allows
users to obtain one CLABSI SIR for their facility, adjusting for all locations reported. Similarly, users can also
obtain one CLABSI SIR for all intensive care units in their facility.
Additionally, the SIR allows for a comparison to the national benchmark from a baseline time period, and can be
used to measure progress from a single point in time. In other words, the SIR permits comparisons between the
number of infections experienced by a facility, group, or state to the number of infections that were predicted
to have occurred based on national data (i.e., baseline data).

5 | P a g e
How is the SIR calculated?
The SIR is calculated by dividing the number of observed infections by the number of predicted infections. The
number of predicted infections is calculated using multivariable regression models generated from nationally
aggregated data during a baseline time period. These models are applied to a facility’s denominator and risk
factor data to generate a predicted number of infections. Please refer to the SIR Guide Supplement on page 14
for more details regarding the models.
In order to enforce a minimum precision criterion, SIRs are currently not calculated when the number of
predicted infections is less than 1.0. This rule was instituted to avoid the calculation and interpretation of
statistically imprecise SIRs, which typically have extreme values.
Calculating the Number of Predicted Infections
The number of predicted infections in NHSN is calculated based on the 2015 national HAI aggregate data and is
adjusted for each facility using variables found to be significant predictors of HAI incidence. NHSN uses either a
logistic regression model or a negative binomial regression model to perform this calculation. Logistic regression
models are used when there is an opportunity for a single outcome for each exposure (e.g., SSI following a
procedure). Negative binomial regression models are used when estimating incidence from a summarized
population (e.g., CLABSIs in a Medical ICU). Examples in applying each model type are provided below.
❖ Example: Logistic Regression Model (SSI)
The logistic regression model is the specific type of model used for surgical site infection risk adjustment. At a
high level, the model uses a set of fixed parameters (adjustment variables) to predict the log-odds of a surgical
site infection following an inpatient procedure. To obtain the total number of predicted SSIs, the following steps
are completed in NHSN:
1. Determine the log-odds for each procedure
2. Convert the log-odds into a probability, or risk of infection
, for each procedure
3. Sum the risk of infections across all procedures in a given timeframe
The sum of the risks from a set of procedures will amount to the total number of predicted infections for that
same set of procedures. Table 1 below shows the risk factors found to be significant for abdominal hysterectomy
(HYST) procedures (Complex 30-Day model) in NHSN. Note that each risk factor’s contribution to the SIR varies,
as represented by the parameter estimate for each factor. Parameter estimates describe the relationship
between the variable and the risk of SSI; positive parameter estimates indicate that the risk of SSI increases with

6 | P a g e
increasing values of the variable. Negative parameter estimates indicate that the risk of SSI decreases with
increasing values of the variable.
Table 1. Risk Factors for SSI HYST: Complex 30-Day Model (2015 Baseline)
The parameter estimates from Table 1 can be plugged into the following general logistic regression formula:
The probability of SSI is calculated using the logistic regression model above, by utilizing the relationship
between the log-odds and the probability (risk). Let’s say we have a patient (Patient 1) who is 32 years old, has
diabetes, and a BMI score of 29. She had an ASA score of 2 and her procedure took place in an oncology
hospital. We can use the model above to plug in these values as shown below:
Factor
Parameter Estimate
P-value
Variable Coding
Intercept
-5.1801
-
-
Diabetes
0.3247
<0.0001
Yes= 1
No= 0
ASA Score
0.4414
<0.0001
1= 1
2= 2
3= 3
4/5= 4
Body Mass Index (BMI)
0.1106
0.0090
≥ 30= 1
< 30= 0
Patient Age
-0.1501
<0.0001
Patient’s age/10
Oncology Hospital
0.5474
0.0005
Oncology hospital= 1
Non-oncology hospital= 0
, where:
α = Intercept
β
i
= Parameter Estimate
X
i
= Value of Risk Factor (Categorical variables= 1 if present, 0 if not present. Refer to
“Variable Coding” column in Table 1 above.)
i = Number of Predictors
7 | P a g e
= -3.9055
The value -3.9055 is the log-odds of SSI for Patient 1. To convert this value into the risk of SSI (), we must use
the logit function below:
Note that this can also be interpreted as a 2.0% risk of SSI for Patient 1. The probability of SSI is calculated for
each procedure and then summed across all procedures to give the total number of predicted SSIs for this
population. Table 2 provides a partial list of 100 hypothetical patients who have undergone this particular
procedure type and demonstrates how the total number of predicted SSIs is calculated.
Table 2. Risk Factors for 100 Patients Undergoing a HYST Procedure (Complex 30-Day model)
Notice in the above table that the probability of SSI is different for each patient, given the risk factors present
during the reported procedure.
The SIR can now be calculated for those 100 procedures as follows:
Patient
Diabetes
ASA score
BMI
Age
Oncology Hospital
SSI Identified?
Probability of SSI
1
Y
2
29
32
Y
1
0.020
2
N
3
35
49
Y
0
0.019
3
N
5
20
51
Y
1
0.026
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
100
N
4
27
27
Y
0
0.037
TOTAL
.
.
.
.
.
8 (observed SSIs)
6.750 (predicted SSIs)

8 | P a g e
❖ Example: Negative Binomial Regression Model
Negative binomial regression models are used to calculate the number of predicted events for CLABSI, MBI-LCBI,
CAUTI, VAE, MRSA bacteremia LabID, and C. difficile (CDI) LabID under the 2015 baseline. Below is a general
formula for a negative binomial regression model.
As an example, Table 3 below represents the negative binomial regression model used to calculate the number
of predicted healthcare facility-onset (HO) CDI LabID events in acute care hospitals under the 2015 baseline.
Table 3. Risk Factors Used in the Acute Care Hospital CDI LabID Event Model
The SIR for C. difficile LabID events in an acute care hospital is calculated on the facility-wide inpatient
(FacWideIN) level for each quarter. More information on the details of the LabID Event SIR calculations can be
found in the SIR Guide Supplement on page 14.
We can input the model details from Table 3 into the general negative binomial regression formula for CDI in
acute care hospitals:
Factor
Parameter Estimate
P-value
Intercept
-8.9463
<0.0001
Inpatient community-onset (CO) admission prevalence rate
0.7339
<0.0001
CDI test type= EIA
-0.1579
<0.0001
CDI test type= NAAT
0.1307
<0.0001
# ICU beds: ≥ 43
0.7465
<0.0001
# ICU beds: 20-42
0.7145
<0.0001
# ICU beds: 10-19
0.6261
<0.0001
# ICU beds: 5-9
0.4394
<0.0001
Oncology hospital (facility type = HOSP-ONC)
1.2420
<0.0001
General acute care hospital (facility type = HOSP-GEN)
0.3740
<0.0001
Total facility bed size
0.0003
<0.0001
CDI LabID surveillance in ED or 24-hour observation location(s)
0.1119
<0.0001
Teaching facility (major, graduate, or undergraduate)
0.0331
0.0028
, where:
α = Intercept
β
i
= Parameter Estimate
X
i
= Value of Risk Factor (Categorical variables: 1 if present, 0 if not present)
i = Number of Predictors

9 | P a g e
# predicted HO CDI =
For most variables shown in parentheses in the equation above, you would replace the variable name (and
therefore, multiply each parameter estimate) with a “1” or “0” depending on whether that factor is present in
your facility (Yes= “1”, No= “0”). The inpatient CO prevalence rate and total number of beds are continuous
variables and should be replaced with the actual values of the inpatient CO prevalence rate and total number of
beds. The last step in the equation is to multiply the resulting value by the appropriate HAI denominator (i.e.,
patient days for MRSA/CDI events, or device days for CLABSI/MBI/CAUTI/VAE). In this example, we multiply by
CDI patient days.
Note: in NHSN, “CDI patient days” refers to the patient days entered on Row 3 of the FacWideIN monthly
denominator forms, for an entire quarter. This value represents that total number of patient days from
all inpatient units within the facility, with the exception of NICUs, well-baby units, and CMS-certified
rehab and psych units.
Let’s walk through an example of calculating the number of predicted CDI events for an acute care hospital for
2015 Q1. The facility in our example has reported 5,000 CDI patient days and 5 healthcare facility-onset CDI
LabID events in 2015 Q1. After running the CDI rate tables in NHSN, the facility records that their 2015 Q1 CO
admission prevalence rate was 1.25 per 100 admissions. The facility was using a NAAT CDI test type, has 5 ICU
beds, is enrolled in NHSN as a children’s non-teaching hospital, and has 100 total beds. The facility has an
Emergency Department and is thus reporting CDI data from this location per NHSN protocol.

10 | P a g e
In our example hospital, the completed formula looks like this:
Because the facility was not using EIA test type, was not a general or oncology hospital, and was not a teaching
hospital, the associated parameters in the model were not met. Therefore, the parameter estimates for each of
those variables were multiplied by 0 and fell out of the equation.
To calculate the CDI LabID SIR, divide the number of observed HO CDI LabID events by the number of predicted
HO CID LabID events. In our example:
11 | P a g e
Finding and Interpreting SIRs in NHSN
What SIR reports are available?
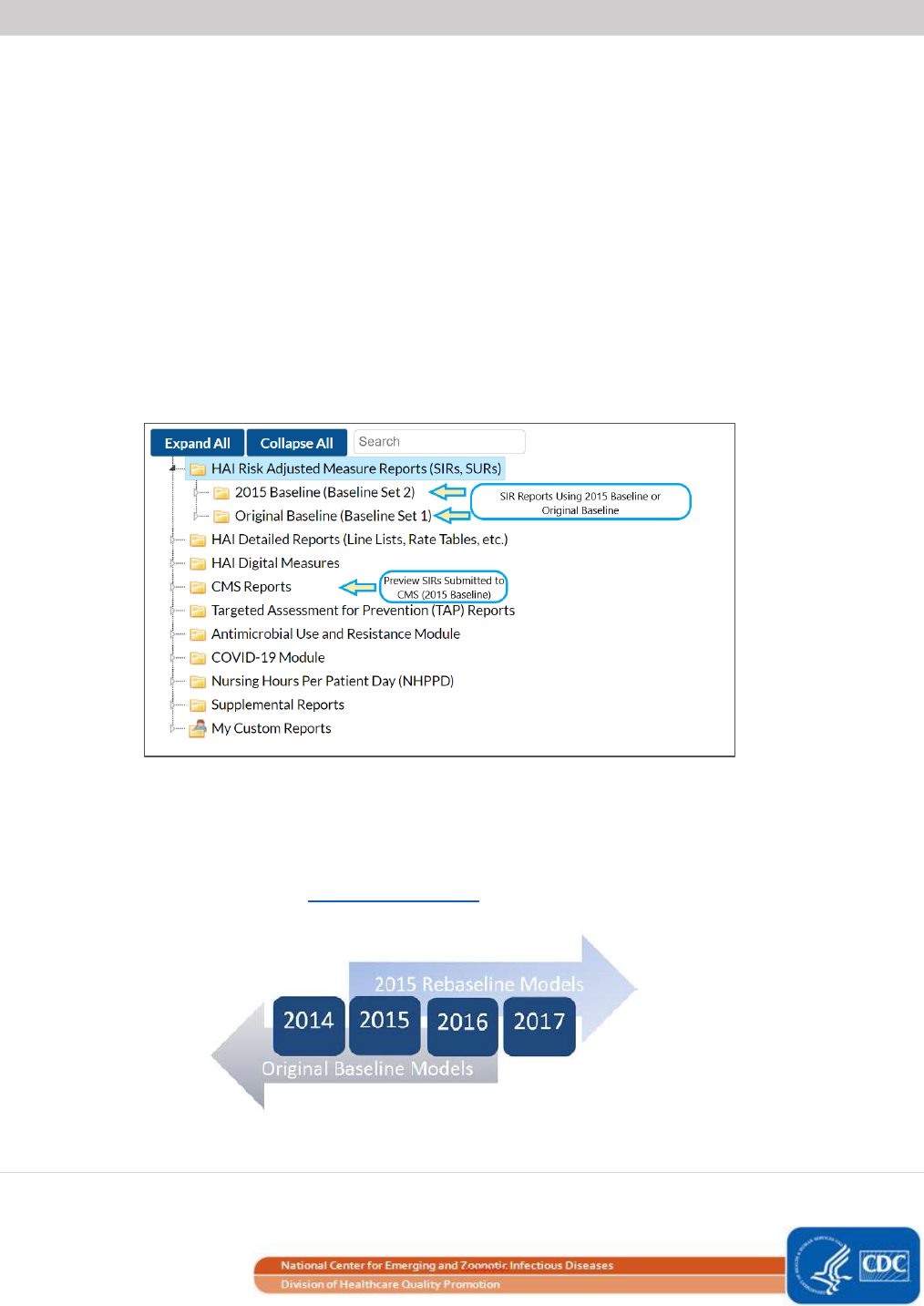
To run analysis reports in NHSN, users must first generate analysis data sets (Analysis > Generate Data Sets).
NHSN recommends users regenerate data sets after entering new data into the application or before creating
new reports. After data sets have been regenerated, users can select Analysis > Reports from the NHSN
homepage to view all of the available reports. SIR Reports can be found under the "HAI Risk Adjusted Measure
Reports" tab, and either the 2015 baseline or the original baseline can be selected. Each baseline folder contains
HAI-specific subfolders with all of the available SIR reports, rate tables, and run charts for the chosen baseline. In
addition, SIR reports are available that mirror the data submitted to the Centers for Medicare & Medicaid
Services (CMS) Quality Reporting Programs. These reports can be found in the analysis folder titled “CMS
Reports”.
SIRs can be generated for data through 2016 using the original NHSN baselines by running reports in the
“Baseline Set 1” reports folder. Data representing a later time period (i.e., starting in January 2017) can only be
analyzed in NHSN using the new 2015 rebaseline models. Year 2016 is the final year of data that can use the
original models to calculate SIRs. See Additional Resources for information about the original SIR baselines.

12 | P a g e
Note: SIRs calculated under the original baseline cannot be directly compared to SIRs calculated under the
updated baseline. Additional information about NHSN Re-baseline can be found here:
https://www.cdc.gov/nhsn/pdfs/training/2017/Dudeck_March21.pdf;
https://www.cdc.gov/nhsn/pdfs/training/2017/Dudeck_March22.pdf.
How do I Interpret the SIRs?
SIR
• If the SIR > 1.0, then more HAIs were observed than predicted, based on the 2015 national aggregate
data.
• If the SIR < 1.0, then fewer HAIs were observed than predicted, based on the 2015 national aggregate
data.
• If the SIR= 1.0, then the same number of HAIs were observed as predicted, based on the 2015 national
aggregate data.
• Remember, the SIR is only calculated when the number of predicted infections is at least 1.0. When the
predicted number of infections is less than 1.0, facilities have a few options for reviewing and
interpreting HAI data in NHSN:
o A longer time period can be included in the SIR calculation in order to reach the threshold of 1.0
predicted infection.
o Infection rates can be used to track internal HAI incidence over time.
o Run the TAP Reports to review the CAD (cumulative attributable difference, which is the
difference between the number of observed infections and the number of predicted infections,
multiplied by the SIR goal). Information and guidance about running TAP reports can be found in
Additional Resources.
P-value
• In the context of the SIR, the p-value is a statistical measure that tells us whether the number of
observed infections is statistically significantly different than the number of predicted infections (i.e.,
whether the SIR is significantly different from 1.0). NHSN calculates p-values using a mid-P exact test.
• Given the typical cutoff value of 0.05, if the p-value ≤ 0.05, we can conclude that the number of
observed infections is statistically significantly different than the number of predicted infections.
• If the p-value > 0.05, then we can conclude that the number of observed infections is not statistically
significantly different than the number of predicted infections.
95% Confidence Interval
• The 95% confidence interval is a statistical range of values in which we have a high degree of confidence
that the true SIR lies.

13 | P a g e
• If the confidence interval does not include the value of 1, then the SIR is significantly different than 1
(i.e., the number of observed infections is significantly different than the number of predicted
infections).
o Example: 95% confidence interval= (0.85, 0.92)
• If the confidence interval includes the value of 1, then the SIR is not significantly different than 1 (i.e.,
the number of observed infections is not significantly different than the number of predicted infections).
o Example: 95% confidence interval= (0.85, 1.24)
• If the SIR is 0.000 (i.e., the observed infection count is 0 and the number of predicted infections is ≥ 1.0),
then the lower bound of the 95% confidence interval will not be calculated.
As an example, let’s take a look at the CLABSI SIR output. Below is a table showing the overall CLABSI SIR for a
hospital during the first quarter of 2015.
• During the first quarter (January– March) of 2015 (“summaryYQ”), there were 5 CLABSIs identified in our
facility (“infCount”), and we observed a total of 1,850 central line days (“numcldays”) from the locations
under surveillance.
• Based on the NHSN 2015 baseline data, 2.365 CLABSIs were predicted (“numPred”) in our facility.
• This results in an SIR of 2.114 (5/2.365), signifying that during this time period, our facility identified
more CLABSIs than were predicted.
• Because the p-value (“SIR_pval”) is above the significance level of 0.05 and the 95% confidence interval
(“sir95ci”) includes the value of 1, we can conclude that our facility’s SIR is not statistically significant; in
other words, our facility did not observe a statistically significantly different number of CLABSIs than
predicted.
When analyzing these data as a Group user, an additional overall SIR will be calculated for all facilities in the
Group. More information about using the Group function in NHSN can be found here:
https://www.cdc.gov/nhsn/group-users/index.html.

14 | P a g e
SIR Guide Supplement: Risk Adjustment Factors Included in the SIR
Calculations, 2015 Baseline
Introduction to the SIR Guide Supplement
The following pages contain information on the risk factors used in the calculation of the number of predicted
events for each HAI and facility type under the 2015 SIR baseline. This information is provided in order to aide in
the interpretation of the SIR calculations produced by NHSN. The tables displayed in this document list the
variables included in each risk adjustment model, as well as parameter estimates and standard errors. Some risk
adjustment variables are broken into different levels, or categories (i.e., categorical variables), while other
variables are treated as continuous variables without any categorization. Standard errors reflect the precision of
the parameter estimate.
• Categorical variables:
Example: “medical school affiliation” in the CAUTI Acute Care Hospital model, page 23
Variables are categorized based on significant differences in HAI risk between the categories. Parameter
estimates reflect the nature of the relationship between the variable and the risk of HAI. In the case of
categorical variables, the risk of HAI in an individual category is compared to the risk of HAI in the “referent”
category. A positive parameter estimate indicates that the risk of HAI in that category (and therefore, the
number of predicted HAIs) is higher compared to the risk of HAI in the referent category. A negative parameter
estimate indicates that the HAI risk in that category is lower compared to the HAI risk in the “referent” category.
• Continuous variables:
Example: “facility bed size” in the CDI Acute Care Hospital model, page 39
Parameter estimates reflect the nature of the relationship between the variable and the risk of HAI (and
therefore, the number of predicted HAIs). For continuous variables, a positive parameter estimate indicates that
the risk of HAI increases as the variable increases, while negative parameter estimates indicate that the risk of
HAI decreases as the variable increases.
• Derived variables:
Example: The proportion of admissions with traumatic and non-traumatic spinal cord dysfunction in the CAUTI IRF
model, page 26
Derived variables are variables created from two or more variables, and may involve summation, division, or
multiplication. They may be categorical or continuous. Parameter estimates are interpreted as above if the
derived variables are categorical or continuous.

15 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
CLABSI – Central Line-Associated Bloodstream Infection
The number of predicted CLABSIs is calculated using a negative binomial regression model (see page 8 above for
more information). Inpatient locations that were previously excluded from the original baseline are now
included in the SIR under the 2015 baseline (e.g., Telemetry Ward, Mixed Acuity Ward). Refer to Table 1 below
for a list of location types included in the Acute Care Hospital CLABSI SIR; if an inpatient location is not included
in the model table below, then data from that location type will be excluded from the SIR due to insufficient
2015 baseline data. In addition, data from Governmental and Non-governmental Public Health Emergency (PHE)
Facilities (facType as HOSP-PHE/G or HOSP-PHE/NG), will also be excluded from the SIR. In cases when the
number of predicted events is less than 1.0, the SIR will not be calculated in NHSN. CLABSI events reported to
NHSN as mucosal barrier injury (MBI-LCBI), or with extracorporeal life support (ECMO), or a ventricular assist
device (VAD) (2019 events and later) are excluded from the numerator of the CLABSI SIR.
The number of predicted CLABSIs calculated under the 2015 baseline is risk adjusted based on the following
variables found to be statistically significant predictors (risk adjustment updated August 2018):
Table 1. CLABSI in Acute Care Hospitals (non-NICU locations)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-7.6325
0.0606
<0.0001
CDC Location Code: Adult Critical Care Units, Oncology
Critical Care Units
Medical Cardiac Critical Care
Surgical Cardiothoracic Critical Care
Medical Critical Care
Medical/Surgical Critical Care
Neurologic Critical Care
Neurosurgical Critical Care
Medical Oncology Critical Care
Medical/Surgical Oncology Critical Care
Pediatric Oncology Critical Care
Surgical Oncology Critical Care
Prenatal Critical Care
Respiratory Critical Care
Surgical Critical Care
0.3257
0.0435
<0.0001
CDC Location Code: Pediatric Critical Care
Pediatric Burn Critical Care
Pediatric Cardiothoracic Critical Care
Pediatric Medical/Surgical Critical Care
Pediatric Medical Critical Care
Pediatric Neurosurgical Critical Care
Pediatric Surgical Critical Care
Pediatric Trauma Critical Care
0.5695
0.0699
<0.0001
CDC Location Code: Burn Critical Care (Adult)
1.4269
0.1125
<0.0001
CDC Location Code: Trauma Critical Care (Adult)
0.6287
0.0835
<0.0001
CDC Location Code: Specialty Care Areas
Inpatient Dialysis
Solid Organ Transplant (adult)
0.3766
0.1304
0.0039

16 | P a g e
Parameter
Parameter Estimate
Standard Error
P-value
Solid Organ Transplant (pediatric)
CDC Location Code: Step-down Units
Adult Step-down Unit
Oncology Step-down Unit
Pediatric Step-down Unit
Step-down Neonatal Nursery (Level II)
0.2155
0.0521
<0.0001
CDC Location Code: Select Adult Wards
Medical Ward
Medical/Surgical Ward
Neurology Ward
Neurosurgical Ward
Surgical Ward
Telemetry Ward
0.1797
0.0427
<0.0001
CDC Location Code: Oncology Wards
ONC General Hematology/Oncology Ward
ONC Pediatric General Hematology/Oncology Ward
ONC Leukemia Ward
ONC Leukemia/Lymphoma Ward
ONC Lymphoma Ward
ONC Solid Tumor Ward
0.3698
0.0550
<0.0001
CDC Location Code: Oncology Stem Cell Transplant
Wards
ONC Hematopoietic Stem Cell Transplant Ward (adult)
ONC Pediatric Hematopoietic Stem Cell Transplant Ward
0.6876
0.0816
<0.0001
CDC Location Code: Pediatric Wards & Nurseries
Pediatric Behavioral Health Ward
Pediatric Burn Ward
Pediatric Medical Ward
Pediatric Medical/Surgical Ward
Pediatric Neurosurgical Ward
Well Baby Nursery (Level I)
Pediatric Neurology Ward
Pediatric Orthopedic Ward
Pediatric Rehabilitation Ward (non-CMS)
Pediatric Surgical Ward
0.1912
0.0704
0.0066
CDC Location Code: All Other Wards
Adult Mixed Acuity
Mixed Age Mixed Acuity
Pediatric Mixed Acuity
Oncology Mixed Acuity
Antenatal Care Ward
Burn Ward
Behavioral Health/Psych Ward
Adolescent Behavioral Health Ward
Ear/Nose/Throat Ward
Gastrointestinal Ward
Gerontology Ward
Genitourinary Ward
REFERENT
-
-

17 | P a g e
* Facility bed size and medical school affiliation are taken from the Annual Hospital Survey.
Note: For data from Specialty Care Areas and Oncology locations, CLABSI events are eligible for inclusion in the SIR
regardless of the type of central line (temporary or permanent). Similarly, total central line days (numcldays) used in the SIR
calculation are summed from temporary and permanent central line days reported. If a CLABSI event is reported from a
month/location with missing denominator data for either temporary or permanent central lines, then the data for that
month/location will be excluded from SIR calculations.
Parameter
Parameter Estimate
Standard Error
P-value
Gynecology Ward
Jail Unit
Labor and Delivery Ward
Labor, Delivery, Recovery, Postpartum Suite (LDRP)
Orthopedic Ward
Plastic Surgery Ward
Postpartum Ward
Pulmonary Ward
Rehabilitation Ward (non-CMS)
Stroke (Acute) Ward
Orthopedic Trauma Ward
Vascular Surgery Ward
Chronic Care Unit
Chronic Behavioral Health/Psychiatric Unit
Inpatient Hospice
Chronic Ventilator Dependent
Chronic Rehabilitation Unit
REFERENT
(continued from
previous page)
-
-
Facility bed size*: ≥ 224 beds
0.2571
0.0471
<0.0001
Facility bed size*: 94 - 223 beds
0.1160
0.0493
0.0187
Facility bed size*: ≤ 93 beds
REFERENT
-
-
Medical school affiliation*: Major
0.2627
0.0211
<0.0001
Medical school affiliation*: Graduate
0.1494
0.0244
<0.0001
Medical school affiliation*:Undergraduate/Non-teaching
REFERENT
-
-
Facility type: (based on NHSN enrollment)
Children’s
Military
Veterans’ Affairs
Women’s
Women’s and Children’s
0.1429
0.0526
0.0066
Facility type: (based on NHSN enrollment)
General Acute Care
Oncology
Orthopedic
Psychiatric
Surgical
REFERENT
-
-

18 | P a g e
Table 2. CLABSI in Acute Care Hospital NICUs (Level II/III, Level III, and Level IV NICU locations
Note: For NICUs, CLABSI events as well as central line days are reported by birthweight category. If a CLABSI event is
reported from a month/location with missing denominator data for one of the birthweight categories, then data for that
month/location will be excluded from SIR calculations.
Table 3. CLABSI in Critical Access Hospitals (CAHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept*
-8.2066
0.1967
<0.0001
* None of the variables investigated were statistically significantly associated with CLABSIs in CAHs. The predicted number
of CLABSI events for CAHs is calculated using the 2015 national CAH CLABSI pooled mean (i.e., intercept-only model).
Table 4. CLABSI in Long-Term Acute Care Hospitals (LTACHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-7.8328
0.1307
<0.0001
Location Type: ICU
0.6716
0.1031
<0.0001
Location Type: Ward
REFERENT
-
-
Facility bed size*: ≥ 45 beds
0.2819
0.0686
<0.0001
Facility bed size*: < 45 beds
REFERENT
-
-
Average length of stay*: ≥ 28 days
0.1481
0.0708
0.0365
Average length of stay*: < 28 days
REFERENT
-
-
Proportion of admissions on a ventilator*: ≥ 0.328
0.3907
0.0971
<0.0001
Proportion of admissions on a ventilator*: ≥ 0.125 and
< 0.328
0.2127
0.0859
0.0133
Proportion of admissions on a ventilator*: < 0.125
REFERENT
-
-
Proportion of admissions on hemodialysis*: ≥ 0.138
0.5785
0.1341
<0.0001
Proportion of admissions on hemodialysis*: ≥ 0.008 and
< 0.138
0.5090
0.1296
<0.0001
Proportion of admissions on hemodialysis*: < 0.008
REFERENT
-
-
* Facility bed size, average length of stay, and admission proportions are taken from the Annual LTACH Survey. Average
length of stay is calculated as: total # of annual patient days / total # of annual admissions.
Table 5. CLABSI in Inpatient Rehabilitation Facilities (IRFs): Free-standing Rehabilitation Hospitals and CMS-
Certified IRF Units Within a Hospital
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-8.6717
0.3579
<0.001
Proportion of admissions with stroke*: ≥ 0.135
0.7707
0.3222
0.0168
Proportion of admissions with stroke*: < 0.135
REFERENT
-
-
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-7.2573
0.0553
<0.0001
Birthweight A: ≤ 750 grams
1.2780
0.0745
<0.0001
Birthweight B: 751-1000 grams
0.9780
0.0791
<0.0001
Birthweight C: 1001-1500 grams
0.4579
0.0843
<0.0001
Birthweight D & E: 1501-2500 grams and > 2500 grams
REFERENT
-
-

19 | P a g e
Parameter
Parameter Estimate
Standard Error
P-value
Proportion of admissions in other non-specific diagnostic
categories*: ≥ 0.197
0.4452
0.2051
0.0300
Proportion of admissions in other non-specific diagnostic
categories*: < 0.197
REFERENT
-
-
* Admission proportions are taken from the Annual IRF Survey. “Other non-specific diagnostic categories” include all other
primary diagnoses not listed specifically on the Annual IRF Survey.
20 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
MBI-LCBI – Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infection
The number of predicted MBI-LCBIs is calculated using a negative binomial regression model (see page 8 above
for more information) and is only available for acute care hospitals. Only CLABSI events reported to NHSN as
mucosal barrier injury (MBI-LCBI) are included in the numerator of the MBI-LCBI SIR. Refer to Table 1 below for a
list of location types included in the Acute Care Hospital MBI-LCBI SIR; if an inpatient location is not included in
the model table below, then data from that location type will be excluded from the SIR due to insufficient 2015
baseline data. In addition, data from Governmental and Non-governmental Public Health Emergency (PHE)
Facilities (facType as HOSP-PHE/G or HOSP-PHE/NG) will also be excluded from the SIR. In cases when the
number of predicted events is less than 1.0, the SIR will not be calculated in NHSN.
*Note: The variables included in the MBI risk adjustment model for acute care hospitals are shown below. The
MBI-LCBI SIR is not submitted to CMS.
The number of predicted MBI-LCBI events calculated under the 2015 baseline is risk adjusted based on the
following variables found to be statistically significant predictors:
Table 1. MBI-LCBI in Acute Care Hospitals
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-10.9284
0.1397
<0.0001
CDC Location Code: Adult Critical Care Units
Burn Critical Care
Medical Cardiac Critical Care
Surgical Cardiothoracic Critical Care
Medical Critical Care
Medical-Surgical Critical Care
Neurologic Critical Care
Neurosurgical Critical Care
Prenatal Critical Care
Respiratory Critical Care
Surgical Critical Care
Trauma Critical Care
-0.5102
0.0938
<0.0001
CDC Location Code: Oncology Critical Care Units
Oncology Medical Critical Care
Oncology Medical-Surgical Critical Care
ONC Pediatric Critical Care
Oncology Surgical Critical Care
2.6269
0.4176
<0.0001
CDC Location Code: Pediatric Critical Care Units
Pediatric Burn Critical Care
Pediatric Surgical Cardiothoracic Critical Care
Pediatric Medical-Surgical Critical Care
Pediatric Medical Critical Care
Pediatric Neurosurgical Critical Care
Pediatric Surgical Critical Care
Pediatric Trauma Critical Care
0.7732
0.1803
<0.0001
CDC Location Code: Step-down Units
Adult Step-down Unit
-0.7252
0.2004
0.0003

21 | P a g e
Parameter
Parameter Estimate
Standard Error
P-value
Step-down Neonatal Nursery (Level II)
Pediatric Step-down Unit
CDC Location Code: Oncology Wards and Step-down
Units
ONC Step-down Unit
ONC General Hematology-Oncology Ward
ONC Hematopoietic Stem Cell Transplant Ward
ONC Leukemia Ward
ONC Leukemia/Lymphoma Ward
ONC Lymphoma Ward
ONC Solid Tumor Ward
3.1224
0.0901
<0.0001
CDC Location Code: Pediatric Oncology Wards
ONC Pediatric General Hematology/Oncology Ward
ONC Pediatric Hematopoietic Stem Cell Transplant Ward
3.1967
0.1710
<0.0001
CDC Location Code: Pediatric Wards
Pediatric Behavioral Health Ward
Pediatric Burn Ward
Pediatric Medical-Surgical Ward
Pediatric Medical Ward
Pediatric Neurosurgical Ward
Pediatric Neurology Ward
Pediatric Orthopedic Ward
Pediatric Rehabilitation Ward (within Hospital)
Pediatric Surgical Ward
1.3335
0.1464
<0.0001
CDC Location Code: All Other Wards
Mixed Age Mixed Acuity Unit
Adult Mixed Acuity Unit
Pediatric Mixed Acuity Unit
Oncology Mixed Acuity Unit
Dialysis Specialty Care Area
Solid Organ Transplant Specialty Care Area
Pediatric Solid Organ Transplant Specialty Care Area
Antenatal Care Ward
Burn Ward
Behavioral Health/Psych Ward
Adolescent Behavioral Health Ward
Ear, Nose, Throat Ward
Gastrointestinal Ward
Gerontology Ward
Genitourinary Ward
Gynecology Ward
Jail Unit
Labor and Delivery Ward
Labor, Delivery, Recovery, Postpartum Suite
Medical Ward
Medical-Surgical Ward
Neurology Ward
Neurosurgical Ward
Well Baby Nursery (Level I)
Orthopedic Ward
REFERENT
-
-

22 | P a g e
Parameter
Parameter Estimate
Standard Error
P-value
Plastic Surgery Ward
Postpartum Ward
Pulmonary Ward
Rehabilitation Ward (within Hospital)
Surgical Ward
Stroke (Acute) Ward
Telemetry Ward
Orthopedic Trauma Ward
Vascular Surgery Ward
Chronic Care Unit
Chronic Behavioral Health/Psych Unit
Inpatient Hospice
Ventilator Dependent Unit
Chronic Rehabilitation Unit
Facility bed size*: ≥ 149 beds
0.5422
0.1389
<0.0001
Facility bed size*: <149 beds
REFERENT
-
-
Medical school affiliation*: Major
0.4113
0.0699
<0.0001
Medical school affiliation*:
Graduate/Undergraduate/Non-teaching
REFERENT
-
-
* Facility bed size and medical school affiliation are taken from the Annual Hospital Survey.

23 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
CAUTI – Catheter-Associated Urinary Tract Infection
The number of predicted CAUTIs is calculated using a negative binomial regression model (see page 8 above for
more information). Previously excluded inpatient locations from the original baseline are included in the SIR
under the 2015 baseline (e.g., Telemetry Ward, Mixed Acuity Ward). Refer to Table 1 below for a list of location
types included in the Acute Care Hospital CAUTI SIR; if an inpatient location is not included in the model table
below, then data from that location type will be excluded from the SIR due to insufficient 2015 baseline data. In
addition, data from Governmental and Non-governmental Public Health Emergency (PHE) Facilities (facType as
HOSP-PHE/G or HOSP-PHE/NG) will also be excluded from the SIR. In cases when the number of predicted
events is less than 1.0, the SIR will not be calculated in NHSN.
The number of predicted CAUTIs calculated under the 2015 baseline is risk adjusted based on the following
variables found to be statistically significant predictors (risk adjustment updated July 2017):
Table 1. CAUTI in Acute Care Hospitals
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-10.2667
0.1618
<0.0001
CDC Location Code: Burn Critical Care
3.3318
0.1580
<0.0001
CDC Location Code: Cardiac Critical Care
2.5703
0.1301
<0.0001
CDC Location Code: Medical Critical Care
2.3834
0.1250
<0.0001
CDC Location Code: Neurologic Critical Care and
Neurosurgical Critical Care
3.3675
0.1285
<0.0001
CDC Location Code: Surgical Critical Care
2.7034
0.1270
<0.0001
CDC Location Code: Trauma Critical Care
3.1104
0.1344
<0.0001
CDC Location Code: Other Critical Care
Surgical Cardiothoracic Critical Care
Medical/Surgical Critical Care
Prenatal Critical Care
Respiratory Critical Care
2.3661
0.1214
<0.0001
CDC Location Code: Oncology Critical Care/Step-down
Oncology Medical Critical Care
Oncology Medical/Surgical Critical Care
Surgical Oncology Critical Care
Pediatric Oncology Critical Care
Oncology Mixed Acuity Unit
Oncology Step-Down Unit
2.2171
0.2239
<0.0001
CDC Location Code: Pediatric Cardiothoracic Critical Care
2.0965
0.2322
<0.0001
CDC Location Code: Other Pediatric Critical Care
Pediatric Burn Critical Care
Pediatric Medical/Surgical Critical Care
Pediatric Medical Critical Care
Pediatric Neurosurgical Critical Care
Pediatric Surgical Critical Care
Pediatric Trauma Critical Care
2.6419
0.1461
<0.0001
CDC Location Code: Mixed Acuity
Adult Mixed Acuity Unit
2.3378
0.1416
<0.0001

24 | P a g e
Parameter
Parameter Estimate
Standard Error
P-value
Pediatric Mixed Acuity Unit
Mixed Age Mixed Acuity Unit
CDC Location Code: Adult Step-down Unit
2.4800
0.1235
<0.0001
CDC Location Code: Pediatric Step-down Unit
Neonatal Step-down Nursery (Level II)
Pediatric Step-down Unit
2.3616
0.5351
<0.0001
CDC Location Code: Solid Organ Transplant
Solid Organ Transplant SCA
Pediatric Solid Organ Transplant SCA
2.3900
0.1979
<0.0001
CDC Location Code: Adult Burn Ward
2.4564
0.3396
<0.0001
CDC Location Code: Behavioral/Psychiatric Ward
3.2503
0.2207
<0.0001
CDC Location Code: Pulmonary Ward
2.5024
0.1664
<0.0001
CDC Location Code: Rehabilitation Ward (non-CMS)
3.3578
0.2700
<0.0001
CDC Location Code: Neurology and Stroke
Neurologic Ward
Neurosurgical Ward
Stroke Ward
2.8223
0.1314
<0.0001
CDC Location Code: Orthopedic Ward
Orthopedic Ward
Orthopedic Trauma Ward
1.9992
0.1300
<0.0001
CDC Location Code: Other Wards
Inpatient Dialysis SCA
Gerontology Ward
Jail Unit
Medical Ward
Telemetry Ward
2.3576
0.1216
<0.0001
CDC Location Code: Other Wards
Ear, Nose, Throat Ward
Gastroenterology Ward
Genitourinary Ward
Medical/Surgical Ward
Plastic Surgery Ward
Surgical Ward
Vascular Surgery Ward
2.2532
0.1210
<0.0001
CDC Location Code: Hematology
General Hematology/Oncology Ward
Hematopoietic Stem Cell Transplant Ward
2.6125
0.1315
<0.0001
CDC Location Code: Pediatric Oncology
Pediatric Hematology/Oncology Ward
Pediatric Hematopoietic Stem Cell Transplant Ward
2.7077
0.2915
<0.0001
CDC Location Code: Adult Oncology Wards
Leukemia Ward
Lymphoma Ward
Leukemia/Lymphoma Ward
Solid Tumor Ward
2.2253
0.2001
<0.0001
CDC Location Code: Pediatric Wards
Adolescent Behavioral Ward
Pediatric Behavioral Ward
1.8899
0.1712
<0.0001

25 | P a g e
Parameter
Parameter Estimate
Standard Error
P-value
Pediatric Burn Ward
Pediatric Medical/Surgical Ward
Pediatric Medical Ward
Pediatric Neurosurgical Ward
Pediatric Neurologic Ward
Pediatric Orthopedic Ward
Pediatric Rehabilitation Ward (non-IRF)
Pediatric Surgical Ward
Well-baby Nursery
CDC Location Code: Chronic Care
Chronic Care Unit
Chronic Behavioral Health/Psychiatric Unit
Chronic Rehabilitation Unit
Inpatient Hospice
Ventilator Dependent Unit
2.7695
0.1855
<0.0001
CDC Location Code: Labor and Delivery, Gynecology
Antenatal Ward
Gynecology Ward
Labor and Delivery Ward
Labor, Delivery, Postpartum Ward
Postpartum Ward
REFERENT
-
-
Medical school affiliation*: Major
0.3744
0.0195
<0.0001
Medical school affiliation*: Graduate
0.1313
0.0220
<0.0001
Medical school affiliation*:Undergraduate/Non-teaching
REFERENT
-
-
Facility bed size*: ≥ 215 beds
0.4901
0.0429
<0.0001
Facility bed size*: 87-214 beds
0.2871
0.0445
<0.0001
Facility bed size*: ≤ 86 beds
REFERENT
-
-
Facility type: (based on NHSN enrollment)
General Acute Care Hospital
Military Hospital
Psychiatric Hospital
Oncology Hospital
Veterans' Affairs Hospital
0.3927
0.1069
0.0002
Facility type: Children's Hospital
0.4888
0.1556
0.0017
Facility type: (based on NHSN enrollment)
Orthopedic Hospital
Surgical Hospital
Women's Hospital
Women's and Children's Hospital
REFERENT
-
-
* Medical school affiliation and facility bed size are taken from the Annual Hospital Survey.

26 | P a g e
Table 2. CAUTI in Critical Access Hospitals (CAHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-7.3337
0.0970
<0.0001
Medical school affiliation*: Undergraduate
1.3191
0.4744
0.0054
Medical school affiliation*: Major/Graduate/Non-
teaching
REFERENT
-
-
* Medical school affiliation is taken from the Annual Hospital Survey.
Table 3. CAUTI in Long-Term Acute Care Hospitals (LTACHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-6.8683
0.0773
<0.0001
Average length of stay*: ≥ 29.33 days
0.5379
0.0837
<0.0001
Average length of stay*: 26.42 – 29.32 days
0.2779
0.0876
0.0015
Average length of stay*: ≤ 26.41 days
REFERENT
-
-
Setting**: Freestanding
0.1700
0.0716
0.0176
Setting**: Within a Hospital
REFERENT
-
-
Location Type: ICU
0.3153
0.1072
0.0033
Location Type: Ward
REFERENT
-
-
* Average length of stay is taken from the Annual LTACH Survey. It is calculated as: total # of annual patient days / total # of
annual admissions.
** LTACH Setting (free-standing vs. within a hospital) is taken from the Annual LTACH Survey.
Table 4. CAUTI in Inpatient Rehabilitation Facilities (IRFs): Free-standing Rehabilitation Hospitals and CMS-
Certified IRF Units Within a Hospital
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-6.8305
0.0848
<0.0001
Setting*: Within a Hospital
0.2897
0.0841
0.0006
Setting*: Freestanding
REFERENT
-
-
Proportion of admissions with traumatic and non-
traumatic spinal cord dysfunction**: ≥ 0.05
0.3603
0.0832
<0.0001
Proportion of admissions with traumatic and non-
traumatic spinal cord dysfunction**: < 0.05
REFERENT
-
-
Proportion of admissions with stroke**: ≥ 0.24
0.2750
0.0798
0.0006
Proportion of admissions with stroke**: < 0.24
REFERENT
-
-
* IRF Setting is taken from the Annual IRF Survey and NHSN enrollment/location mapping data. “Within a hospital” includes
CMS-certified IRF units mapped as locations within a hospital, as well as Rehabilitation hospitals enrolled as unique facilities
in NHSN in which the facility indicated “healthcare facility-based” on their annual IRF survey.
** Proportion of annual admissions with primary diagnoses are taken from the Annual IRF Survey and are calculated as: #
of admissions with the primary diagnosis (stroke, or traumatic/non-traumatic spinal cord dysfunction) / total # of annual
admissions.

27 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
VAE – Ventilator-Associated Events
A. Total VAE in Long-Term Acute Care Hospitals (LTACHs)
The number of predicted VAE events is calculated using a negative binomial regression model (see page 8 above
for more information). Separate VAE SIRs are available for “Total VAE” and “IVAC Plus”. The “Total VAE” SIR
includes events identified as ventilator-associated condition (VAC), infection-related ventilator-associated
complication (IVAC), and possible ventilator-associated pneumonia (pVAP). In cases when the number of
predicted events is less than 1.0, the SIR will not be calculated in NHSN.
The number of predicted “Total VAE” events calculated under the 2015 baseline is risk adjusted based on the
following variables found to be statistically significant predictors of Total VAE incidence:
Table 1. Total VAE in Long-Term Acute Care Hospitals (LTACHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-8.3689
0.3361
<0.0001
Facility bed size
†
: ≥ 32 beds
0.4645
0.1562
0.0030
Facility bed size
†
: < 32 beds
REFERENT
-
-
Proportion of admissions on hemodialysis*: > 0.11
-0.4098
0.1190
0.0006
Proportion of admissions on hemodialysis*: ≤ 0.11
REFERENT
-
-
Proportion of admissions on ventilator*: > 0.18
0.9313
0.1813
<0.0001
Proportion of admissions on ventilator*: ≤ 0.18
REFERENT
-
-
Location type: ICU
0.4118
0.1598
0.0099
Location type: Ward
REFERENT
-
-
Average length of stay**: ≥ 25 days
1.0940
0.2602
<0.0001
Average length of stay**: < 25 days
REFERENT
-
-
† Facility bed size is taken from the Annual LTACH Survey.
* Proportion of annual admissions on a ventilator (or hemodialysis) is taken from the Annual LTACH Survey. It is calculated
as: number of admissions on a ventilator (or hemodialysis) / total # of annual admissions.
**Average length of stay is taken from the Annual LTACH Survey. It is calculated as: # annual patient days/ # annual
admissions.
B. Infection-related Ventilator-Associated Complication (IVAC) Plus in Long-Term
Acute Care Hospitals (LTACHs)
The number of predicted VAE events is calculated using a negative binomial regression model (see page 8 above
for more information). Separate VAE SIRs are available for “Total VAE” and “IVAC Plus”. The “IVAC Plus” SIR
includes events identified as IVAC and possible ventilator-associated pneumonia (pVAP). In cases when the
number of predicted events is less than 1.0, the SIR will not be calculated in NHSN.
The number of predicted “IVAC Plus” events calculated under the 2015 baseline is risk adjusted based on the
following variables found to be statistically significant predictors of “IVAC Plus” incidence:

28 | P a g e
Table 1. IVAC Plus in Long-Term Acute Care Hospitals
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-9.9593
0.5891
< 0.0001
Facility bed size
†
: ≥ 32 beds
1.1201
0.3633
0.0020
Facility bed size
†
: < 32 beds
REFERENT
-
-
Proportion of admissions on a ventilator*: > 0.18
0.7130
0.3151
0.0236
Proportion of admissions on a ventilator*: ≤ 0.18
REFERENT
-
-
Average length of stay**: ≥ 25 days
0.8166
0.4157
0.0495
Average length of stay**: < 25 days
REFERENT
-
-
† Facility bed size is taken from the Annual LTACH Survey.
* Proportion of annual admissions on a ventilator is taken from the Annual LTACH Survey. It is calculated as: number of
admissions on a ventilator / total # of annual admissions.
** Average length of stay is taken from the Annual LTACH Survey. It is calculated as: total # of annual patient days / total #
of annual admissions.
C. Total VAE in Acute Care Hospitals (ACHs)
The number of predicted VAE events is calculated using a negative binomial regression model (see page 8 above
for more information). Separate VAE SIRs are available for “Total VAE” and “IVAC Plus”. The “Total VAE” SIR
includes events identified as Ventilator-Associated Condition (VAC), Infection-related Ventilator-Associated
Complication (IVAC), and Possible Ventilator-Associated Pneumonia (PVAP). Refer to Table 1 below for a list of
location types included in the Acute Care Hospital Total VAE SIR; if an inpatient location is not included in the
model table below, then data from that location type will be excluded from the SIR due to insufficient 2015
baseline data. In addition, data from Governmental and Non-governmental Public Health Emergency (PHE)
Facilities (facType as HOSP-PHE/G or HOSP-PHE/NG) will also be excluded from the SIR. In cases when the
number of predicted events is less than 1.0, the SIR will not be calculated in NHSN.
The number of predicted “Total VAE” events calculated under the 2015 baseline is risk adjusted based on the
following variables found to be statistically significant predictors of Total VAE incidence:
Table 1. Total VAE in Acute Care Hospitals (ACHs)
Parameter
Parameter
Estimate
Standard
Error
P-value
Intercept
-6.8748
0.1407
<0.0001
CDC Location Code: Adult Critical Care Units,
Oncology Critical Care Units
Oncology Medical Critical Care
Oncology Surgical Critical Care
Oncology Medical-Surgical Critical Care
Prenatal Critical Care
Respiratory Critical Care
0.5009
0.1810
0.0057
CDC Location Code: Surgical Cardiothoracic Critical
Care
0.9418
0.0862
<.0001

29 | P a g e
Parameter
Parameter
Estimate
Standard
Error
P-value
CDC Location Code: Medical-Surgical Critical Care
1.0161
0.0822
<.0001
CDC Location Code: Adult Critical Care Units
Burn Critical Care
Medical Cardiac Critical Care
Medical Critical Care
Neurologic Critical Care
Neurosurgical Critical Care
Surgical Critical Care
1.1140
0.0820
<.0001
CDC Location Code: Adult Mixed Acuity Unit
1.3225
0.1296
<.0001
CDC Location Code: Trauma Critical Care
1.4320
0.0882
<.0001
CDC Location Code: Step-down Units
Adult Step-down Unit
Oncology Step-down Unit
0.4096
0.1060
0.0001
CDC Location Code: Wards, Solid Organ Transplant
Specialty Care Area
Antenatal Care Ward
Behavioral Health/Psychology Ward
Burn Ward
Ear, Nose, Throat Ward
Gastrointestinal Ward
Genitourinary Ward
Gerontology Ward
Gynecology Ward
Jail Unit
Labor and Delivery Ward
Labor, Delivery, Recovery, Postpartum Suite
Medical Ward
Medical-Surgical Ward
Neurology Ward
Neurosurgical Ward
Oncology Leukemia Ward
Oncology Lymphoma Ward
Oncology Leukemia/Lymphoma Ward
Oncology Solid Tumor Ward
Oncology Hematopoietic Stem Cell Transplant
Ward
Oncology General Hematology-Oncology Ward
Ophthalmology Ward
Orthopedic Ward
Orthopedic Trauma Ward
REFERENT
-
-

30 | P a g e
Parameter
Parameter
Estimate
Standard
Error
P-value
Plastic Surgery Ward
Postpartum Ward
Pulmonary Ward
Rehabilitation Ward (within Hospital)
School Infirmary
Stroke (Acute) Ward
Surgical Ward
Telemetry Ward
Vascular Surgery Ward
Solid Organ Transplant Specialty Care Area
Facility bed size
*
: 85-129 beds
0.1591
0.0787
0.0433
Facility bed size
*
: 130-425 beds
0.2513
0.0679
0.0002
Facility bed size
*
: 426-526 beds
0.5123
0.0716
<.0001
Facility bed size
*
: ≥ 527 beds
0.6471
0.0706
<.0001
Facility bed size
*
: ≤ 84 beds
REFERENT
-
-
Medical School Affiliation*: Major
0.2905
0.0239
<.0001
Medical School Affiliation*:
Graduate/Undergraduate
0.1395
0.0240
<0.0001
Medical School Affiliation*: Non-teaching
REFERENT
-
-
Facility Type (based on NHSN enrollment): GEN-VA
General Acute Care Hospital
Veterans’ Affairs Hospital
0.2154
0.0987
0.0290
Facility Type (based on NHSN enrollment): Other
Military Hospital
Psychiatry Hospital
Oncology Hospital
Orthopedic Hospital
Surgical Hospital
Women’s Hospital
Women’s and Children’s Hospital
REFERENT
-
-
* Facility bed size and medical school affiliation are taken from the Annual ACH Survey
Table 2. Summary of Risk Factors in the Total VAE Model for Other Facility Types
* None of the variables investigated were statistically significantly associated with Total VAE in CAHs. These facilities will
have the predicted number of events calculated using the 2015 national pooled mean (i.e., intercept-only model).
^ Insufficient data were reported to NHSN. Therefore, SIRs are not available for Total VAE in IRFs.
Facility Type
Risk Factors
Critical Access Hospitals (CAH)
Intercept-only model*
Inpatient Rehabilitation Facilities (IRF)
No SIR available
^

31 | P a g e
D. Infection-related Ventilator-Associated Complication (IVAC) Plus in Acute Care
Hospitals (ACHs)
The number of predicted VAE events is calculated using a negative binomial regression model (see page 8 above
for more information). Separate VAE SIRs are available for “Total VAE” and “IVAC Plus”. The “IVAC Plus” SIR
includes events identified as IVAC and Possible Ventilator-Associated Pneumonia (PVAP). Refer to Table 1 below
for a list of location types included in the Acute Care Hospital IVAC Plus SIR; if an inpatient location is not
included in the model table below, then data from that location type will be excluded from the SIR due to
insufficient 2015 baseline data. In addition, data from Governmental and Non-governmental Public Health
Emergency (PHE) Facilities (facType as HOSP-PHE/G or HOSP-PHE/NG) will also be excluded from the SIR. In
cases when the number of predicted events is less than 1.0, the SIR will not be calculated in NHSN.
The number of predicted “IVAC Plus” events calculated under the 2015 baseline is risk adjusted based on the
following variables found to be statistically significant predictors of “IVAC Plus” incidence:
Table 1. IVAC Plus in Acute Care Hospitals (ACHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-7.4627
0.0925
< 0.0001
CDC Location Code: Adult Critical Care Units
Burn Critical Care
Neurologic Critical Care
Neurosurgical Critical Care
Surgical Critical Care
1.1747
0.0922
<.0001
CDC Location Code: Adult Critical Care Units, Oncology
Critical Care Units
Medical Cardiac Critical Care
Medical Critical Care
Medical-Surgical Critical Care
Oncology Medical Critical Care
Oncology Surgical Critical Care
Oncology Medical-Surgical Critical Care
Prenatal Critical Care
Respiratory Critical Care
Surgical Cardiothoracic Critical Care
0.9092
0.0889
<.0001
CDC Location Code: Trauma Critical Care
1.5429
0.0984
<.0001
CDC Location Code: Adult Mixed Acuity Unit
1.2291
0.1779
<.0001
CDC Location Code: Wards, Specialty Care Areas, Step-
down Units
Antenatal Care Ward
Behavioral Health/Psychology Ward
Burn Ward
Ear, Nose, Throat Ward
Gastrointestinal Ward
Genitourinary Ward
REFERENT
-
-

32 | P a g e
Parameter
Parameter Estimate
Standard Error
P-value
Gerontology Ward
Gynecology Ward
Jail Unit
Labor and Delivery Ward
Labor, Delivery, Recovery, Postpartum Suite
Medical Ward
Medical-Surgical Ward
Neurology Ward
Neurosurgical Ward
Oncology Leukemia Ward
Oncology Lymphoma Ward
Oncology Leukemia/Lymphoma Ward
Oncology Solid Tumor Ward
Oncology Hematopoietic Stem Cell Transplant Ward
Oncology General Hematology-Oncology Ward
Ophthalmology Ward
Orthopedic Ward
Orthopedic Trauma Ward
Plastic Surgery Ward
Postpartum Ward
Pulmonary Ward
Rehabilitation Ward (within Hospital)
School Infirmary
Stroke (Acute) Ward
Surgical Ward
Telemetry Ward
Vascular Surgery Ward
Solid Organ Transplant Specialty Care Area
Adult Step-down Unit
Oncology Step-down Unit
Facility bed size
*
: 290-425 beds
0.1540
0.0370
<.0001
Facility bed size
*
: 426-526 beds
0.4058
0.0433
<.0001
Facility bed size
*
: >=527 beds
0.5079
0.0385
<.0001
Facility bed size
*
: ≤ 289 beds
REFERENT
-
-
Medical School Affiliation*: Major
0.3157
0.0354
<.0001
Medical School Affiliation*: Graduate/Undergraduate
0.1630
0.0362
<.0001
Medical School Affiliation*: Non-teaching
REFERENT
-
-
* Facility bed size and medical school affiliation are taken from the Annual ACH Survey
Table 2. Summary of Risk Factors in the IVAC Plus Model for Other Facility Types
Facility Type
Risk Factors
Critical Access Hospitals (CAH)
No SIR Available
^
Inpatient Rehabilitation Facilities (IRF)
No SIR Available
^
^ Insufficient data were reported to NHSN. Therefore, SIRs are not available for ‘IVAC Plus’ in CAHs or IRFs.

33 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
SSI – Surgical Site Infections
The number of predicted SSI events is calculated using a logistic regression model (see page 5 above for more
information). The SSI SIR is calculated for facilities who enroll in NHSN as acute care hospitals or critical access
hospitals. Data from Governmental and Non-governmental Public Health Emergency (PHE) Facilities (facType as
HOSP-PHE/G or HOSP-PHE/NG) are excluded from the SIR. Under the 2015 SIR baseline, procedures and
associated SSI events occurring in adult and pediatric patients are modeled separately. There are three SSI SIR
models available for inpatient adult procedures (and associated SSIs) and two models available for inpatient
pediatric procedures (and associated SSIs). Please see Table 1 below for a summary of the SSI SIR models. Under
the 2015 SIR baseline, procedures, regardless of closure methods, are included in the SIR calculation, as long as
the inclusion criteria listed below are met and none of the exclusion criteria apply.
Table 1. Summary of SSI Models
SSI SIR Model
Inclusion Criteria
Patient Population
All SSI SIR Model
• Includes only inpatient procedures
• Includes Superficial, Deep & Organ/Space SSIs
• Superficial & Deep Incisional SSIs limited to primary
incisional SSIs only
• Includes SSIs identified on admission, readmission & via
post-discharge surveillance
• Procedures in
adult patients
• Procedures in
pediatric patients
Complex
Admission/Readmission
(A/R) SSI Model
• Includes only inpatient procedures
• Includes only Deep Incisional Primary SSIs & Organ/Space
SSIs
• Includes only SSIs identified on Admission/Readmission
to facility where procedure was performed
• Used for the annual CDC publication of national
benchmarks
• Procedures in
adult patients
• Procedures in
pediatric patients
Complex 30-Day SSI
model (used for CMS
IPPS)
• Includes only in-plan, inpatient COLO and HYST
procedures in adult patients (i.e., ≥ 18 years of age)
• Includes only Deep Incisional Primary SSIs and
Organ/Space SSIs with an event date within 30 days of
the procedure
• Includes SSIs regardless of detection method
• Used only for CMS IPPS reporting and for public reporting
on the CMS Care Compare website
• Procedures in
adult patients
Exclusion Criteria
In addition to the above inclusion criteria, there is also a list of exclusion criteria that applies to all the SSI SIR
models. This list is often referred to as the universal exclusion criteria. The list of exclusion criteria applies to
both procedures and the associated SSI events. Often the reason for excluding procedures and SSI events from
the SIR calculation is due to potential data quality issues. It is important that facilities review their data for
quality assurance and to determine the reason for exclusion from the SIR calculation.
Note: When a procedure is excluded from the denominator, the associated SSI event is excluded from the
numerator.

34 | P a g e
Table 2. Universal Procedure/SSI Event Exclusions
General Exclusions
Gender= ‘Other’
Outpatient procedures and resulting SSIs
Present at time of surgery (PATOS) is ‘Yes’
SSIs that are reported as superficial incisional secondary (SIS) or deep incisional secondary (DIS)
Exclusions due to potential data quality issues or outliers
Age at the time of procedure is greater than 109 years
Closure technique is missing
ASA score is missing
Gender is missing
Adult patients ≥ 18 years: if BMI is less than 12 or greater than 60*
Pediatric patients < 18 years: if BMI less than 10.49 or greater than 65.79**
Procedure duration less than 5 minutes
Procedure duration is greater than IQR5 (please see Table 4 in the SSI Section for more information)
Facility-level Exclusions
Data from ambulatory surgery centers (ASCs) and long-term acute care hospitals (LTACHs)
Medical affiliation is missing or medical affiliation is ‘Y’ and medical type is missing (from Annual Facility
Survey)
Number of beds is missing (from Annual Facility Survey)
*This BMI exclusion applies to all procedures on adult patients in all 3 SSI models (All SSI, Complex A/R, Complex 30-Day).
**This BMI exclusion applies to all procedures on pediatric patients, in both applicable SSI models (All SSI and Complex
A/R). CDC Growth Charts are used to assess BMI in pediatric patients, calculated using height, weight, age, and gender.
Additional clarification on the BMI exclusion rule for pediatric procedures: Although there are BMI thresholds for
procedures performed on pediatric patients (10.49-65.79), there is an additional level of consideration made for the
biological plausibility of a given BMI using the patient’s age and gender. After applying the BMI outlier exclusion rule, we
review the BMIs for the remaining pediatric procedures to determine if they are biologically plausible based on the
patient’s age and gender. So essentially, we take age and gender into consideration along with the calculated BMI. Only
procedures in which the patient’s BMI meets the inclusion rule (10.49-65.79), and in which the patient’s BMI is biologically
plausible based on age and gender, are included in the SIR. The determination of biologically plausible BMIs is made using
the macro available at this site: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
Predictive Risk Factors by SSI Models
The number of predicted events calculated under the 2015 baseline for SSI is risk adjusted based on the
following variables found to be statistically significant predictors of SSIs. The following tables (3a-3f) list the
factors included in each procedure-specific model, grouped by the three SSI models outlined above. In some
procedure-specific models, the interaction of age and gender is considered as a single factor. It is listed as age-
gender interaction. In cases when the number of predicted events is less than 1.0, the SIR will not be calculated
in NHSN.
Note: Parameter estimates are shown for colon (COLO) and abdominal hysterectomy (HYST) procedures under
the Complex 30-Day Model used for the CMS Hospital Inpatient Prospective Payment System (IPPS). Full model
details for all procedures under the All-SSI Model and the Complex A/R Model are available here:
https://www.cdc.gov/nhsn/ps-analysis-resources/sirguide-ssimodels-508.xlsx

35 | P a g e
Table 3a. Colon Procedures, Complex 30-Day Model
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-3.6601
0.0678
<0.0001
Diabetes: Yes
0.0821
0.0303
0.0066
Diabetes: No
REFERENT
-
-
ASA score: 1, 2, 3/4/5
0.3028
0.0237
<0.0001
Gender: Male
0.1036
0.0225
<0.0001
Gender: Female
REFERENT
-
-
Age (Patient’s age/10)
-0.1396
0.0075
<0.0001
BMI: ≥ 30
0.1259
0.0234
<0.0001
BMI: < 30
REFERENT
-
-
Closure technique: Other (non-Primary)
0.2383
0.0494
<0.0001
Closure technique: Primary
REFERENT
-
-
Oncology Hospital: Yes
0.5437
0.0937
<0.0001
Oncology Hospital: No
REFERENT
-
-
Table 3b. Abdominal Hysterectomy Procedures, Complex 30-Day Model
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-5.1801
0.1057
< 0.0001
Diabetes: Yes
0.3247
0.0605
<0.0001
Diabetes: No
REFERENT
-
-
ASA score: 1, 2, 3, 4/5
0.4414
0.0350
<0.0001
BMI: ≥ 30
0.1106
0.0423
0.0090
BMI: < 30
REFERENT
-
-
Age (Patient’s age/10)
-0.1501
0.0180
<0.0001
Oncology Hospital: Yes
0.5474
0.1578
0.0005
Oncology Hospital: No
REFERENT
-
-
Procedure Duration Outliers
The IQR5, also called the procedure duration cutoff point, is used as an indicator of an extreme outlier for
procedure durations when calculating the SSI SIRs. The IQR5 is calculated as five times the interquartile range
(Q1-Q3) above the 75th percentile. For example, if the interquartile range is 30 minutes, and the 75th percentile
is 100 minutes, the IQR5 would be calculated as: 100 + (30*5) = 250 minutes. Procedures with a duration greater
than the IQR5 were excluded from the baseline data and will be excluded from all SSI SIR calculations for your
facility.
Tables 3c to 3f have been moved from this section and are now available to view
(with details) in the addendum of the SIR guide, here: https://www.cdc.gov/nhsn/ps-
analysis-resources/sirguide-ssimodels-508.xlsx

36 | P a g e
Table 4. IQR5 Values, in Minutes, for NHSN Operative Procedures, Adult and Pediatric Patients
NHSN Operative Procedure
IQR5 (in minutes)
IQR5 (in hours and minutes)
Minutes
Hours
Minutes
AAA
1116
18
36
AMP
300
5
0
APPY
210
3
30
AVSD
471.5
7
51.5
BILI
1295
21
35
BRST
777
12
57
CARD
1001
16
41
CBGB
847
14
7
CBGC
847
14
7
CEA
376
6
16
CHOL
346
5
46
COLO
697
11
37
CRAN
904
15
4
CSEC
170
2
50
FUSN
874
14
34
FX
532
8
52
GAST
489
8
9
HER
521
8
41
HPRO
349
5
49
HTP
1355
22
35
HYST
547
9
7
KPRO
316
5
16
KTP
670
11
10
LAM
687
11
27
LTP
1243
20
43
NECK
1796
29
56
NEPH
774
12
54
OVRY
594
9
56
PACE
311
5
11
PRST
737
12
17
PVBY
850
14
10
REC
1136
18
56
RFUSN
1129
18
49
SB
856
14
16
SPLE
1073
17
53
THOR
721
12
1
THYR
506
8
26
VHYS
506
8
26
VSHN
378
6
18
XLAP
724
12
4

37 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
Methicillin-resistant Staphylococcus aureus (MRSA) Bacteremia Laboratory-Identified
Events
The number of predicted MRSA bacteremia LabID events is calculated using a negative binomial regression
model (see page 8 above for more information). For most settings, the MRSA bacteremia SIR is only calculated
on the facility-wide inpatient, or FacWideIN, level, and cannot be calculated for any individual location (note:
CMS-designated inpatient rehabilitation units within a hospital will receive a separate SIR). Data from
Governmental and Non-governmental Public Health Emergency (PHE) Facilities (facType as HOSP-PHE/G or
HOSP-PHE/NG) are excluded from the SIR. In cases when the number of predicted events is less than 1.0, the SIR
will not be calculated in NHSN. The SIRs for MRSA bacteremia include only healthcare facility-onset (HO), non-
duplicate MRSA blood LabID events in the numerator. Information on which events are counted in the
numerator of the MRSA bacteremia SIR can be found here: http://www.cdc.gov/nhsn/pdfs/ps-analysis-
resources/mrsacdi_tips.pdf.
The number of predicted events calculated under the 2015 baseline for MRSA bacteremia is risk adjusted based
on the following variables found to be statistically significant predictors of MRSA bacteremia incidence:
Notes for Acute Care Hospitals: MRSA LabID SIRs for acute care hospitals can only be calculated at the quarter-
level or higher. This is because two of the risk factors involving the community-onset prevalence rate require that
all community-onset data have been entered for an entire quarter. The quarter’s community-onset prevalence
rates, both inpatient and outpatient, are used to calculate the number of predicted events for the SIR.
Table 1. MRSA Bacteremia in Acute Care Hospitals
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-11.3759
0.1167
<0.0001
Inpatient community-onset prevalence rate*: > 0.037 per
100 admissions
0.3650
0.0286
<0.0001
Inpatient community-onset prevalence rate*: ≤ 0.037 per
100 admissions
REFERENT
-
-
Average length of stay**: ≥ 5.1 days
0.2787
0.0343
<0.0001
Average length of stay**: 4.3-5.0 days
0.0955
0.0341
0.0050
Average length of stay**: 0-4.2 days
REFERENT
-
-
Medical school affiliation
‡
: Major
0.2585
0.0334
<0.0001
Medical school affiliation
‡
: Graduate/undergraduate
0.1166
0.0345
0.0007
Medical school affiliation
‡
: Non-teaching
REFERENT
-
-
Facility type: Oncology Hospital (HOSP-ONC)
1.1894
0.2085
<0.0001
Facility type: General Acute Care Hospital (HOSP-GEN)
0.4355
0.0897
<0.0001
Facility type: Other Specialty Hospital
REFERENT
-
-
Number of ICU beds
‡
: ≥ 45
0.5650
0.0898
<0.0001
Number of ICU beds
‡
: 21-44
0.4599
0.0899
<0.0001
Number of ICU beds
‡
: 11-20
0.3394
0.0922
0.0002
Number of ICU beds
‡
: 7-10
0.4720
0.0993
<0.0001
Number of ICU beds
‡
: 0-6
REFERENT
-
-
38 | P a g e
Table 1, continued. MRSA Bacteremia in Acute Care Hospitals
Parameter
Parameter Estimate
Standard Error
P-value
Outpatient community-onset prevalence rate ED/24-hour
Observation Unit
^
: > 0.032 per 100 encounters
0.3476
0.0336
<0.0001
Outpatient community-onset prevalence rate ED/24-hour
Observation Unit
^
: > 0 and ≤ 0.032 per 100 encounters
0.1048
0.0330
0.0015
Outpatient community-onset prevalence rate ED/24-hour
Observation Unit
^
: 0 per 100 encounters, or no applicable
locations
REFERENT
-
-
* Inpatient community-onset prevalence is calculated as the # of inpatient community-onset MRSA blood events, divided by
total admissions x 100. (i.e., MRSA_admPrevBldCount /numadms * 100).
** Average length of stay is taken from the Annual Hospital Survey. It is calculated as: total # of annual patient days / total #
of annual admissions.
‡
Medical school affiliation and number of ICU beds are taken from the Annual Hospital Survey.
^ Emergency department (ED)/24-hour observation unit prevalence rate combines MRSA bacteremia data from all EDs
and/or 24-hour observation units into a single, de-duplicated prevalence rate. This rate is calculated as the # of unique
community-onset MRSA blood events that occurred in an ED or 24-hour observation unit / total encounters * 100. (i.e.,
MRSA_EDOBSprevCount / numTotencounters * 100). NOTE: If you do not have an ED or 24-hour observation location that
meets the NHSN location definition and thus are not reporting MRSA bacteremia data from these locations, the number of
predicted events will be risk adjusted using the referent level of this variable.
Table 2. MRSA Bacteremia in Critical Access Hospitals
(CAHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept*
-10.7795
0.2025
<0.0001
* MRSA LabID SIRs for CAHs can be calculated for any aggregate of time (month, quarter, half-year, or year). None of the
variables investigated were statistically significantly associated with healthcare facility-onset MRSA bacteremia in CAHs. The
predicted number of events for CAHs will be calculated using the 2015 national CAH MRSA bacteremia pooled mean (i.e.,
intercept-only model).
Table 3. MRSA Bacteremia in Long-Term Acute Care Hospitals (LTACHs)
* MRSA LabID SIRs for LTACHs can be calculated for any aggregate of time (month, quarter, half-year, or year).
** Percent of annual admissions on a ventilator is taken from the Annual LTACH Survey. It is calculated as: # admissions on
a ventilator / total # of annual admissions x 100 (i.e., numAdmvent /numAdmitsSurv * 100).
Table 4. MRSA Bacteremia in Inpatient Rehabilitation Hospitals
(IRFs): Free-standing Rehabilitation Hospitals
and CMS-Certified IRF Units Within a Hospital
Parameter
Parameter Estimate
Standard Error
P-value
Intercept*
-10.8703
0.0890
<0.0001
* MRSA LabID SIRs for IRFs can be calculated for any aggregate of time (month, quarter, half-year, or year). None of the
variables investigated were statistically significantly associated with healthcare facility-onset MRSA bacteremia in IRFs.
Free-standing IRFs and CMS-certified IRF units within a hospital will have the predicted number of events calculated using
the 2015 national IRF MRSA bacteremia pooled mean (i.e., intercept-only model).
Parameter
Parameter Estimate
Standard Error
P-value
Intercept*
-9.3095
0.0936
<0.0001
Percent of admissions on ventilator**
0.0160
0.0027
<0.0001
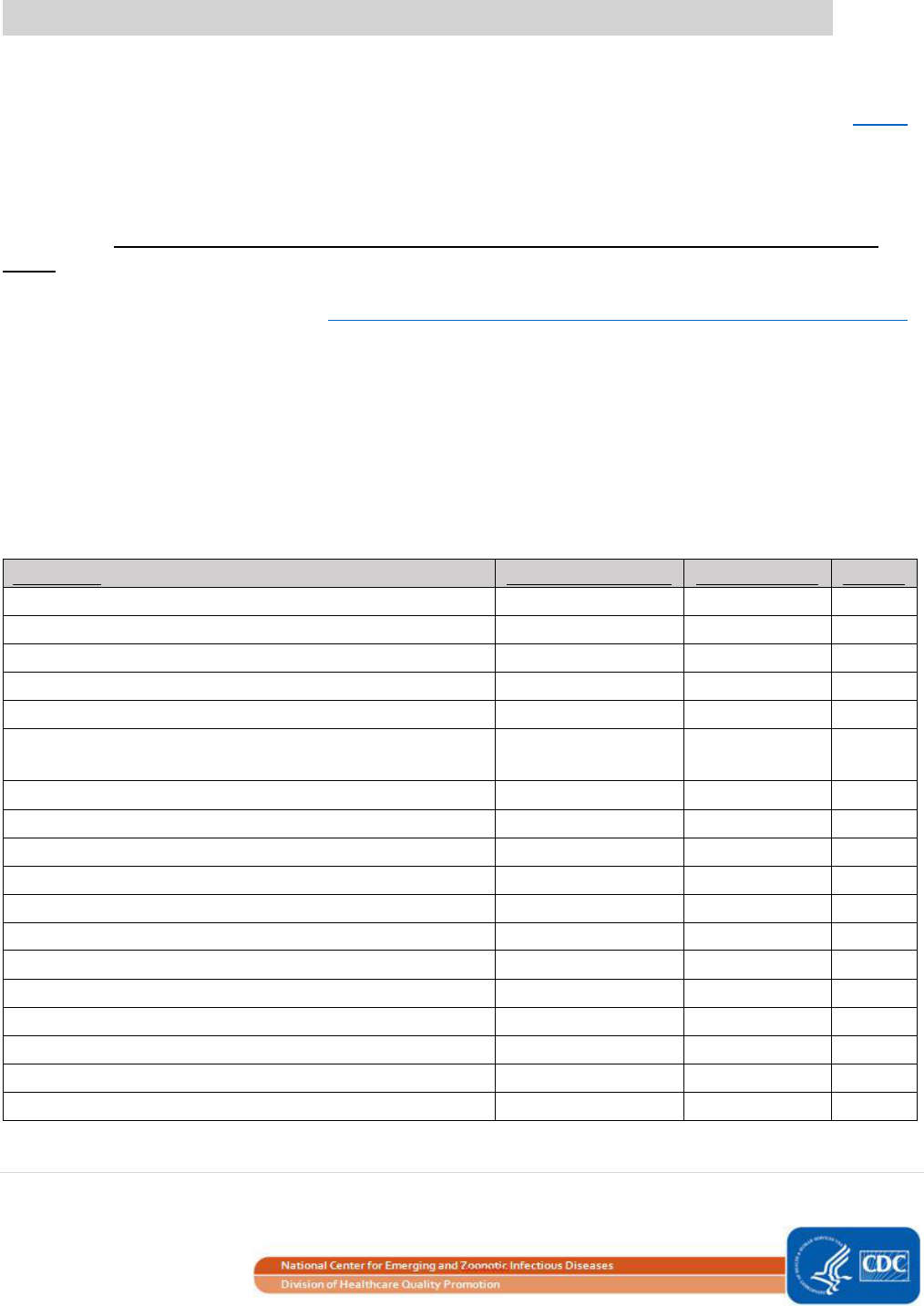
39 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
Clostridioides difficile (CDI) Laboratory-Identified Events
The number of predicted CDI LabID events is calculated using a negative binomial regression model (see page 8
above for more information). For most settings, the CDI SIR is only calculated on the facility-wide inpatient, or
FacWideIN, level, and cannot be calculated for any individual location (note: CMS-designated inpatient
rehabilitation units within a hospital will receive a separate SIR). Data from Governmental and Non-
governmental Public Health Emergency (PHE) Facilities (facType as HOSP-PHE/G or HOSP-PHE/NG) are excluded
from the SIR. In cases when the number of predicted events is less than 1.0, the SIR will not be calculated in
NHSN. The FacWideIN SIRs for CDI include only incident, healthcare facility-onset (HO), non-duplicate C. difficile
LabID events in the numerator. Information on which events are counted in the numerator of the FacWideIN
and IRF Unit CDI SIR can be found here: http://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/mrsacdi_tips.pdf.
For all facility types, the CDI LabID SIR can only be calculated at the quarter-level or higher. Monthly SIRs cannot
be calculated due to certain risk factors used in each of the models that require complete data entry for a
quarter (e.g., CDI test type is reported on the FacWideIN and IRF unit’s MDRO denominator form on the 3rd
month of each quarter).
The number of predicted events calculated under the 2015 baseline for CDI is risk adjusted based on the
following variables found to be statistically significant predictors of CDI incidence:
Table 1. CDI in Acute Care Hospitals
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-8.9463
0.0523
<0.0001
Inpatient community-onset prevalence rate*
0.7339
0.0181
<0.0001
CDI test type
+
: EIA
-0.1579
0.0246
<0.0001
CDI test type
+
:
NAAT
0.1307
0.0219
<0.0001
CDI test type
+
: OTHER
REFERENT
-
-
Medical school affiliation
‡
: Major, graduate, or
undergraduate
0.0331
0.0111
0.0028
Medical school affiliation
‡
: Non-teaching
REFERENT
-
-
Number of ICU beds
‡
: ≥ 43
0.7465
0.0412
<0.0001
Number of ICU beds
‡
: 20- 42
0.7145
0.0395
<0.0001
Number of ICU beds
‡
: 10-19
0.6261
0.0396
<0.0001
Number of ICU beds
‡
: 5-9
0.4394
0.0420
<0.0001
Number of ICU beds
‡
: 0-4
REFERENT
-
-
Facility type: Oncology Hospital (HOSP-ONC)
1.2420
0.0765
<0.0001
Facility type: General Acute Care Hospital (HOSP-GEN)
0.3740
0.0342
<0.0001
Facility type: Other Specialty Hospital
REFERENT
-
-
Facility bed size
‡
0.0003
0.0000
<0.0001
Reporting from ED or 24-hour observation unit
^
: YES
0.1119
0.0179
<0.0001
Reporting from ED or 24-hour observation unit
^
: NO
REFERENT
-
-
* Inpatient community-onset (CO) prevalence is calculated as the # of inpatient CO CDI events, divided by total admissions x
100 (i.e., cdif_admPrevCOCount /numCdifadms * 100). The prevalence rate for an entire quarter is used in the risk

40 | P a g e
Table 1 Footnotes continued:
adjustment. An SIR cannot be calculated for any quarter that has an outlier inpatient CO prevalence rate, defined as greater
than 2.6 CO events per 100 admissions.
+
CDI test type is reported on the FacWideIN MDRO denominator form on the 3
rd
month of each quarter.
-Starting in 2018 Q1, CDI test type is categorized as:
Nucleic acid amplification test (NAAT): This includes NAAT, GDH + NAAT, and GDH + EIA + NAAT.
Enzyme immunoassay (EIA) for toxin: This includes EIA for toxin, GDH antigen + EIA for toxin, and NAAT + EIA
Other: This includes all other CDI test types, including the selection of “Other” and associated free-text entry.
-Prior to 2018 Q1, CDI test type was categorized as: (refer to 2018 NHSN protocol changes for details)
Nucleic acid amplification test (NAAT): This includes NAAT, GDH + NAAT, GDH + EIA + NAAT, and NAAT + EIA.
Enzyme immunoassay (EIA) for toxin: This includes EIA for toxin, and GDH antigen + EIA for toxin.
Other: This includes all other CDI test types, including the selection of “Other” and associated free-text entry.
‡
Medical school affiliation, number of ICU beds, and facility bed size are taken from the Annual Hospital Survey.
^ If your facility has a designated Emergency Department (ED) or 24-hour observation location meeting the standard NHSN
definitions, these locations should be mapped, included in your facility’s monthly reporting plan for LabID events, and have
appropriate outpatient LabID data reported to NHSN. If you do not have an ED or 24-hour observation location and thus are
not reporting CDI data from these locations, your hospital’s # predicted events will be risk adjusted using the referent value
for this variable.
Table 2. CDI in Critical Access Hospitals (CAHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-8.4180
0.0879
<0.0001
Inpatient community-onset prevalence rate*: > 0
0.7207
0.1108
<0.0001
Inpatient community-onset prevalence rate*: 0
REFERENT
-
-
* Inpatient community-onset (CO) prevalence rate is calculated as: # of inpatient CO CDI events, divided by total admissions
x 100. (i.e., cdif_admPrevCOCount / numCdifadms * 100). The prevalence rate for an entire quarter is used in the risk
adjustment.
Table 3. CDI in Long-Term Acute Care Hospitals (LTACHs)
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-7.3345
0.0507
<0.0001
Inpatient community-onset prevalence rate*: > 0
0.3683
0.0493
<0.0001
Inpatient community-onset prevalence rate*: 0
REFERENT
-
-
Percent of admissions on a ventilator
‡
: ≥ 27.1%
0.3116
0.0478
<0.0001
Percent of admissions on a ventilator
‡
: ≥ 18% to < 27.1%
0.1463
0.0590
0.0131
Percent of admissions on a ventilator
‡
: < 18%
REFERENT
-
-
CDI test type
^
: NAAT or OTHER
0.1607
0.0444
0.0003
CDI test type
^
: EIA
REFERENT
-
-
Percent of single occupancy rooms
+
: ≤ 77%
0.0963
0.0425
0.0235
Percent of single occupancy rooms
+
: >77%
REFERENT
-
-
* Inpatient community-onset prevalence is calculated as the # of inpatient community-onset CDI events, divided by total
admissions * 100. (i.e., cdif_admPrevCOCount / numCdifadms * 100). The prevalence rate for an entire quarter is used in
the risk adjustment.
‡
Percent of annual admissions on a ventilator is taken from the Annual LTACH Survey. It is calculated as: # admissions on a
ventilator / total # annual admissions x 100. (i.e., numAdmVent / numAdmitsSurv * 100).

41 | P a g e
Table 3 Footnotes continued:
^ CDI test type is reported on the FacWideIN MDRO denominator form on the 3
rd
month of each quarter.
-Starting in 2018 Q1, CDI test type is categorized as:
Nucleic acid amplification test (NAAT) or Other: This includes NAAT, GDH + NAAT, GDH + EIA + NAAT, and all other
(non-EIA) CDI test types, including the selection of “Other” and associated free-text entry.
Enzyme immunoassay (EIA) for toxin: This includes EIA for toxin, GDH antigen + EIA for toxin, and NAAT + EIA.
-Prior to 2018 Q1, CDI test type was categorized as: (refer to 2018 NHSN protocol changes for details)
Nucleic acid amplification test (NAAT) or Other: This includes NAAT, GDH + NAAT, GDH + EIA + NAAT, NAAT + EIA,
and all other (non-EIA) CDI test types, including the selection of “Other” and associated free-text entry.
Enzyme immunoassay (EIA) for toxin: This includes EIA for toxin, and GDH antigen + EIA for toxin.
+
Percent of beds located in single occupancy rooms is taken from the Annual LTACH Survey. It is calculated as: # of single
occupancy rooms / total number of beds x 100. (i.e., numSingOccRm / numbeds * 100).
Table 4. CDI in Inpatient Rehabilitation Facilities (IRFs): Free-standing Rehabilitation Hospitals and CMS-
Certified IRF Units Within a Hospital
Parameter
Parameter Estimate
Standard Error
P-value
Intercept
-8.4475
0.0689
<0.0001
CDI test type
^
: NAAT
0.2921
0.0534
<0.0001
CDI test type
^
: OTHER
0.2163
0.0747
0.0038
CDI test type
^
: EIA
REFERENT
-
-
CMS-certified IRF Unit within a hospital
0.2188
0.0495
<0.0001
Free-standing HOSP-REHAB with reported community-
onset CDI events
0.4168
0.0803
<0.0001
Free-standing HOSP-REHAB with zero reported
community-onset CDI events
REFERENT
-
-
Percent of admissions with orthopedic conditions*:
≤ 23.9%
0.2015
0.0427
<0.0001
Percent of admissions with orthopedic conditions*:
> 23.9%
REFERENT
-
-
Percent of admissions with traumatic and non-traumatic
spinal cord dysfunction*: > 5.2%
0.1657
0.0437
0.0002
Percent of admissions with traumatic and non-traumatic
spinal cord dysfunction*: ≤ 5.2%
REFERENT
-
-
Percent of admissions with stroke*: ≤ 23.8%
0.1965
0.0444
<0.0001
Percent of admissions with stroke*: > 23.8%
REFERENT
-
-
^ CDI test type is reported on the FacWideIN or IRF Unit’s MDRO denominator form on the 3
rd
month of each quarter.
-Starting with 2018 Q1, CDI test type is categorized as:
Nucleic acid amplification test (NAAT): This includes NAAT, GDH + NAAT, and GDH + EIA + NAAT.
Enzyme immunoassay (EIA) for toxin: This includes EIA for toxin, GDH antigen + EIA for toxin, and NAAT + EIA.
Other: This includes all other CDI test types, including the selection of “Other” and associated free-text entry.
-Prior to 2018 Q1, CDI test type was categorized as: (refer to 2018 NHSN protocol changes for details)
Nucleic acid amplification test (NAAT): This includes NAAT, GDH + NAAT, GDH + EIA + NAAT, and NAAT + EIA.
Enzyme immunoassay (EIA) for toxin: This includes EIA for toxin, and GDH antigen + EIA for toxin.
Other: This includes all other CDI test types, including the selection of “Other” and associated free-text entry.
^ Percent of annual admissions with primary diagnoses are taken from the Annual IRF Survey, and calculated as the # of
admissions with the primary diagnosis / total # of annual admissions x 100.

42 | P a g e
Using an Intercept-Only Model to Calculate the Number of Predicted Events
Example: MRSA Bacteremia LabID Event
Several regression models from the 2015 national baseline are “intercept-only models”. For example, none of
the investigated variables were found to have a significant association with the incidence of healthcare facility-
onset (HO) MRSA bacteremia in critical access hospitals or inpatient rehabilitation facilities. Therefore, the
number of predicted events is calculated by applying the following intercept-only formula:
Let’s say a critical access hospital had 1,400 total patient days during a select time period. The number of
predicted events would be calculated as:
Because the number of predicted events is less than 1.0, an SIR will not be calculated for this facility and time
period in NHSN.

43 | P a g e
Additional Resources
➢ Information about Transitioning to 2015 SIR Baselines:
NHSN Rebaseline webpage: https://www.cdc.gov/nhsn/2015rebaseline/
Introduction to the NHSN Re-baseline – March 2017
https://www.cdc.gov/nhsn/pdfs/training/2017/Dudeck_March21.pdf
The NHSN Re-Baseline: In Depth – March 2017
https://www.cdc.gov/nhsn/pdfs/training/2017/Dudeck_March22.pdf
➢ Original SIR Baselines for Acute Care Hospitals:
CLABSI (original baseline = 2006-2008): https://www.cdc.gov/nhsn/PDFs/dataStat/2009NHSNReport.pdf
CAUTI (original baseline = 2009): https://www.cdc.gov/nhsn/PDFs/NHSNReport_DataSummaryfor2009.pdf
SSI (original baseline = 2006-2008): https://www.cdc.gov/nhsn/PDFs/pscManual/SSI_ModelPaper.pdf
MRSA bacteremia and CDI LabID event (original baseline= 2010-2011):
https://www.cdc.gov/nhsn/pdfs/mrsa-cdi/riskadjustment-mrsa-cdi.pdf
December 2010 Special Edition NHSN Newsletter - Introduction to SIR (original baseline):
https://www.cdc.gov/nhsn/pdfs/newsletters/nhsn_nl_oct_2010se_final.pdf
➢ Original SIR Baselines for Long-term Acute Care Hospitals (LTACHs) and Inpatient
Rehabilitation Facilities (IRFs):
CLABSI/CAUTI in LTACHs, and CAUTI in IRFs (original baseline = 2013):
https://www.cdc.gov/nhsn/xls/reportdatatables/nhsn-2013-report.xlsx
➢ NHSN Analysis Trainings & Other Resources:
A comprehensive guide to NHSN’s SSI SIR, including risk factors used in the SIR calculations under the 2015
baseline: https://www.cdc.gov/nhsn/ps-analysis-resources/sirguide-ssimodels-508.xlsx
Analysis Resources, Trainings, and NHSN Data Dictionary:
https://www.cdc.gov/nhsn/ps-analysis-resources/index.html
Targeted Assessment for Prevention (TAP) General Information: https://www.cdc.gov/hai/prevent/tap.html

44 | P a g e
Quick Reference Guides: How to run and interpret NHSN reports (including SIR and TAP reports):
https://www.cdc.gov/nhsn/ps-analysis-resources/reference-guides.html
Troubleshooting CLABSI and CAUTI SIRs:
https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/clabsicauti_sirtroubleshooting.pdf
Troubleshooting SSI SIRs: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/ssi-sir_tips.pdf
Troubleshooting MRSA and CDI LabID Event SIRs:
https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/mrsacdi_tips.pdf
Information related to SIRs used for the Centers for Medicare and Medicaid Services (CMS) Quality Reporting
Programs: https://www.cdc.gov/nhsn/cms/index.html
NHSN Annual Hospital Survey: https://www.cdc.gov/nhsn/forms/57.103_pshospsurv_blank.pdf
• Instructions for NHSN Annual Hospital Survey: https://www.cdc.gov/nhsn/forms/instr/57_103-toi.pdf
NHSN Annual LTACH Survey: https://www.cdc.gov/nhsn/forms/57.150_ltacfacsurv_blank.pdf
• Instructions for NHSN Annual LTACH Survey: https://www.cdc.gov/nhsn/forms/instr/toi-57.150-ltac.pdf
NHSN Annual IRF Survey: https://www.cdc.gov/nhsn/forms/57.151_rehabfacsurv_blank.pdf
• Instructions for NHSN Annual IRF Survey: https://www.cdc.gov/nhsn/forms/instr/toi-57.151-irf.pdf
NHSN Location Mapping: https://www.cdc.gov/nhsn/pdfs/pscmanual/15locationsdescriptions_current.pdf
Keys to Success with NHSN Data: https://www.cdc.gov/nhsn/ps-analysis-resources/keys-to-success.html

45 | P a g e
ADDENDUM TO THE NHSN GUIDE TO THE SIR
Hospital Outpatient Department (HOPD)
Outpatient Procedure Component (OPC)

46 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
Hospital Outpatient Department (HOPD) Procedure/SSI SIR Model
The number of predicted SSI events is calculated using a logistic regression model (see page 5 above for more
information). The SSI SIR is calculated for facilities who enroll in NHSN as acute care hospitals or critical access hospitals.
Data from Governmental and Non-governmental Public Health Emergency (PHE) Facilities (facType as HOSP-PHE/G or
HOSP-PHE/NG) are excluded from the SIR. Under the 2015 SIR baseline, procedures and associated SSI events occurring
in adult and pediatric patients are modeled separately. There is only one SSI SIR Model available for the hospital
outpatient procedures (and associated SSIs). Please see Table 1 below for a summary of the SSI SIR model. Under the
2015 SIR baseline, procedures, regardless of closure methods, are included in the SIR calculation, as long as the inclusion
criteria listed below are met and none of the exclusion criteria apply.
Table 1. Summary of SSI Models
SSI SIR Model
Inclusion Criteria
Patient Population
All SSI SIR Model
• Includes only hospital outpatient procedures
• Includes Superficial, Deep & Organ/Space SSIs
• Superficial & Deep Incisional SSIs limited to primary
incisional SSIs only
• Includes SSIs identified on admission, readmission &
via post-discharge surveillance
• Procedures in
adult patients
• Procedures in
pediatric patients
Exclusion Criteria
The list of exclusion criteria is the same as those listed for the inpatient SSI –Surgical Site Infections-Hospital Inpatient
Procedures Models on page 27. The only difference is that this model includes outpatient procedures and excludes
inpatient procedures.
Predictive Risk Factors for HOPD All SSI SIR Model
The number of predicted events calculated under the 2015 baseline for HOPD SSI is risk adjusted based on the following
variables found to be statistically significant predictors of SSIs. The following tables (2a and 2b) list the factors included
in each procedure category for adults and pediatrics.
Table 2a. Predictive Risk Factors from the hospital outpatient procedure department (HOPD) data: All SSI Logistic
Regression Model, Adults ≥ 18 years of age^
NHSN Operative Procedure
Risk Factor(s) - All SSI SIR Model, Adults
APPY
Intercept-only model
‡
AVSD
Medical school affiliation*, procedure duration
BRST
Wound class, medical school affiliation*, procedure duration, BMI
CHOL
Diabetes, hospital bed size*, procedure duration
COLO
Procedure duration
FUSN
Spinal level
FX
Procedure duration
HER
Gender, wound class, hospital bed size*, procedure duration, BMI

47 | P a g e
Table 2a, Continued^
NHSN Operative Procedure
Risk Factor(s) - All SSI SIR Model, Adults
HPRO
Emergency, hospital bed size*, procedure duration
HYST
Emergency, age, procedure duration, oncology hospital,
KPRO
Medical school affiliation*, age
LAM
Diabetes, medical school affiliation*, procedure duration
OVRY
Intercept-only model
‡
PACE
Intercept-only model
‡
THYR
Intercept-only model
‡
VHYS
Intercept-only model
‡
XLAP
age
* These risk factors are taken from the Annual Facility Survey.
^ SIRs are not available for procedure categories that had insufficient data (i.e., < 50 procedures and <1 SSI event) that were
reported to NHSN during the baseline period.
‡ None of the variables investigated were statistically significantly associated with SSI risk in these procedure categories.
As a result, the overall pooled mean will be used in the SIR calculation (i.e., intercept-only model).
Table 2b. Predictive Risk Factors from the hospital outpatient procedure department (HOPD) data: All SSI Logistic
Regression Model, Pediatrics <18 years of age*
NHSN Operative Procedure
Risk Factor(s) - All SSI SIR Model, Adults
FX
Procedure duration
HER, (age >=2)
BMI
* SIRs are not available for procedure categories that had insufficient data (i.e., < 50 procedures and <1 SSI event) that were
reported to NHSN during the baseline period.
HOPD Procedure Duration Outliers
Please see table 4 on page 49.

48 | P a g e
Risk Adjustment Factors Included in the SIR Calculation: 2015 Baseline
Outpatient Procedure Component Surgical Site Infections (OPC SSI)
The number of predicted SSI events is calculated using a logistic regression model (see page 5 above for more
information). The OPC SSI SIR is calculated for facilities who enroll in NHSN as Ambulatory Surgery Centers (ASC). Data
from Governmental and Non-governmental Public Health Emergency (PHE) Facilities (facType as HOSP-PHE/G or HOSP-
PHE/NG) are excluded from the SIR. Under the 2015 SIR baseline, procedures and associated SSI events occurring in
adult outpatients are modeled separately in this new component. There is one SIR model available for outpatient adult
procedures (and associated SSIs). Please see Table 1 below for a summary of the OPC SSI SIR model.
Table 1. Summary of OPC SSI Model
OPC SSI SIR Model
Inclusion Criteria
Patient Population
All SSI SIR Model
• Includes only ambulatory surgery centers procedures
• Includes Superficial, Deep & Organ/Space SSIs
• Superficial & Deep Incisional SSIs limited to primary
incisional SSIs only
• Includes SSIs identified on active and passive surveillance
• Procedures in
adult patients
Exclusion Criteria
In addition to the above inclusion criteria, there is also a list of exclusion criteria that applies to the OPC All SSI SIR
model. Similar to the PSC, the list of exclusion criteria applies to both procedures and the associated SSI events. Often
the reason for excluding procedures and SSI events from the SIR calculation is due to potential data quality issues. It is
important that facilities review their data for quality assurance and to determine the reason for exclusion from the SIR
calculation.
Note: When a procedure is excluded from the denominator, the associated SSI event is excluded from the numerator.
Table 2. Procedure Exclusions specific to OPC
General Exclusions
Gender= ‘Other’
Inpatient and HOPD procedures and resulting SSIs
SSIs that are reported as superficial incisional secondary (SIS) or deep incisional secondary (DIS)
Exclusions due to potential data quality issues or outliers
Age at the time of procedure is greater than 109 years
Gender is missing
Adult patients ≥ 18 years: if BMI is less than 12 or greater than 60
Procedure duration less than 5 minutes
Procedure duration is greater than IQR5 (please see Table 4 in the OPC SSI Section for more information)
Predictive Risk Factors in OPC All SSI SIR Model
The number of predicted events, calculated under the 2015 baseline for SSI, is risk adjusted based on the following
variables found to be statistically significant predictors of SSIs. The following Table 3 lists the factors included in each
procedure with the OPC All SSI model SIR outlined above. Procedure categories that do not have an SIR available will be

49 | P a g e
revaluated in the future with baseline data to calculate an SIR. In cases when the number of predicted events is less than
1.0, the SIR will not be calculated in NHSN.
Table 3. Predictive Risk Factors from the OPC All SSI Logistic Regression Model, Adults ≥ 18 years of age*
NHSN Operative Procedure
Risk Factor(s) - All SSI SIR Model, Pediatrics
BRST
age, anesthesia, BMI
HER
age, BMI, procedure duration
KPRO
Intercept-only model
‡
LAM
Intercept-only model
‡
*SIRs are not available for procedure categories that had insufficient data (i.e., < 1000 procedures and <1 SSI event) that were
reported to NHSN during the baseline period.
‡ None of the variables investigated were statistically significantly associated with SSI risk in these procedure categories. As a result,
the overall pooled mean will be used in the SIR calculation (i.e., intercept-only model).
Procedure Duration Outliers
The IQR5, also called the procedure duration cutoff point, is used as an indicator of an extreme outlier for procedure
durations when calculating the SSI SIRs. The IQR5 is calculated as five times the interquartile range (Q1-Q3) above the
75th percentile. For example, if the interquartile range is 30 minutes, and the 75th percentile is 100 minutes, the IQR5
would be calculated as: 100 + (30*5) = 250 minutes. Procedures with a duration greater than the IQR5 were excluded
from the baseline data and will be excluded from all SSI SIR calculations for your facility. This list of procedure IQR5
apply to both the HOPD Procedure/SSI SIR and the OPC SIR Models.
Table 4. IQR5 Values, in Minutes, for NHSN Operative Procedures, Adult Outpatient Procedures
NHSN Operative Procedure
IQR5 (in minutes)
IQR5 (in hours and minutes)
Minutes
Hours
Minutes
AMP
197
3
17
APPY
153
2
33
AVSD
308
5
8
BILI
300
5
0
BRST
355
5
5
CEA
477
7
57
CHOL
223
3
43
COLO
524
8
44
CSEC
166
2
46
FUSN
392
6
32
FX
326
5
26
GAST
326
5
26
HER
249
4
9
HPRO
255
4
15
HYST
452
7
32
KPRO
273
4
33
LAM
307
5
7
NECK
384
6
24
NEPH
296
4
46
OVRY
388
6
28
PACE
228
3
48

50 | P a g e
Table 4, Continued
NHSN Operative Procedure
IQR5 (in minutes)
IQR5 (in hours and minutes)
Minutes
Hours
Minutes
PRST
340
5
40
PVBY
627
10
27
REC
228
3
48
RFUSN
542
9
2
SB
669
11
9
SPLE
604
10
4
THOR
414
6
54
THYR
334
8
26
VHYS
433
5
34
VSHN
244
4
4
XLAP
345
5
45
Outpatient Procedure Component Surgical Site Infections (OPC SSI)
ASC Surveillance for SSI Events: https://www.cdc.gov/nhsn/opc/ssi/index.html
